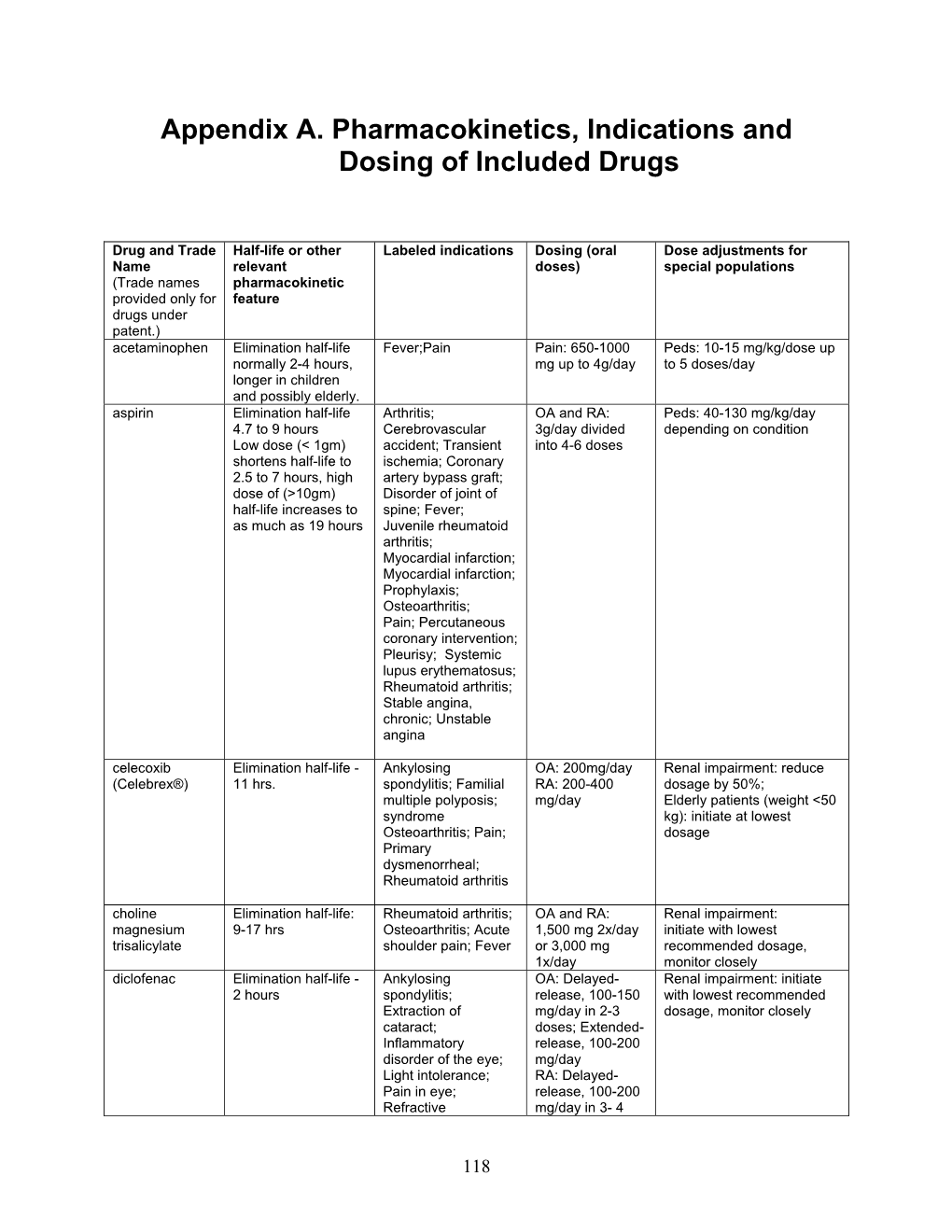
Appendix A. Pharmacokinetics, Indications and Dosing of Included Drugs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pharmacokinetics of Salicylic Acid Following Intravenous and Oral Administration of Sodium Salicylate in Sheep
animals Article Pharmacokinetics of Salicylic Acid Following Intravenous and Oral Administration of Sodium Salicylate in Sheep Shashwati Mathurkar 1,*, Preet Singh 2 ID , Kavitha Kongara 2 and Paul Chambers 2 1 1B, He Awa Crescent, Waikanae 5036, New Zealand 2 School of Veterinary Sciences, College of Sciences, Massey University, Palmerston North 4474, New Zealand; [email protected] (P.S.); [email protected] (K.K.); [email protected] (P.C.) * Correspondence: [email protected]; Tel.: +64-221-678-035 Received: 13 June 2018; Accepted: 16 July 2018; Published: 18 July 2018 Simple Summary: Scarcity of non-steroidal anti-inflammatory drugs (NSAID) to minimise the pain in sheep instigated the current study. The aim of this study was to know the pharmacokinetic parameters of salicylic acid in New Zealand sheep after administration of multiple intravenous and oral doses of sodium salicylate (sodium salt of salicylic acid). Results of the study suggest that the half-life of the drug was shorter and clearance was faster after intravenous administration as compared to that of the oral administration. The minimum effective concentration required to produce analgesia in humans (16.8 µL) was achieved in sheep for about 0.17 h in the current study after intravenous administration of 100 and 200 mg/kg body weight of sodium salicylate. However, oral administration of these doses failed to achieve the minimum effective concentration as mentioned above. This study is of significance as it adds valuable information on pharmacokinetics and its variation due to breed, species, age, gender and environmental conditions. -

SBARD – Lithium Nsaids
SBARD in Relation to Potentially Dangerous Interaction Between Lithium and Anti- Inflammatory Drugs A number of medication incidents have been reported recently concerning the prescribing and administration of non-steroidal anti-inflammatory drugs S (NSAIDs) to patients on lithium. Situation Co-administration of a NSAID or cyclo-oxygenase (COX)-2 inhibitor, with lithium increases the risk of lithium toxicity In the last 6 months (Oct.11-Mar.12) there have been 3 Datix incident reports concerning the prescription of a NSAID for in-patients stabilised on lithium. B In each case the NSAID was prescribed “as required”, which increases the risk Background of lithium toxicity due to irregular administration with no increase in lithium monitoring frequency. NSAIDs and COX-2 inhibitors reduce the renal excretion of lithium via their A action on renal prostaglandins, resulting in increased plasma lithium levels. The level of increase is unpredictable (from 10-400%), and the onset of effect is Assessment variable (few days to several months). Risks are increased in patients with impaired renal function, renal artery stenosis or heart failure, and who are dehydrated or on a low salt diet. Co-prescription of NSAIDs or COX-2 inhibitors with lithium is not an absolute R contra-indication, but should be undertaken with extreme caution and only Recommendation when clinically essential. The risk of lithium toxicity is increased with “as required” or intermittent dosing (e.g. when used for menorrhagia), when the effects are less predictable – regular dosing -

Aspirin Therapy in Primary Prevention of ASCVD
Aspirin Therapy in Primary Prevention of ASCVD ✖ The use of aspirin in primary prevention of atherosclerotic cardiovascular disease (ASCVD) has faced increasing controversy. ✖ A recently published four-trial series evaluating the use of aspirin therapy in the primary prevention of ASCVD has yielded potential challenges to decades-old research as reflected in the current U.S. Preventive Services PROBLEM Task Force guidelines. • In three of the four trials, aspirin failed to show benefit of primary cardiovascular prevention, while suggesting potential harm including higher all-cause mortality and major hemorrhage with reduced disability- free survival. • A fourth trial showed modest reduction in serious vascular events which were “largely counterbalanced” by the observed rate of major bleeding events within the trial. ✔ Low-dose aspirin SHOULD NOT be routinely administered for primary prevention of ASCVD to individuals >70 years of age and those at increased risk of bleeding GENERALLY NO SOLUTION irrespective of age (risk may outweigh benefit), or <40 years of age (insufficient data for determining risk-to-benefit). OCCASIONALLY YES ✔ Low-dose aspirin MAY be considered for primary prevention of ASCVD among adults aged 40-70 years who possess higher ASCVD risk but remain at low probability for bleeding events. LOW-DOSE ASPIRIN USE IN PRIMARY PREVENTION OF ASCVD Study Patient Population Benefit Harm (Aspirin vs. Placebo) ASCEND Diabetes without ASCVD iIn MACE hRisk of major bleeding 8.5% vs. 9.6% 4.1% vs. 3.2% (rate ratio 0.88, CI 0.79-0.97) (rate ratio 1.29, CI 1.09-1.52) ASPREE Elderly: ≥ 70 years of age No reduction all-cause mortality hRisk of major hemorrhage OR 5.9% vs. -

Associations of Regular Glucosamine Use with All-Cause and Cause
Epidemiology Ann Rheum Dis: first published as 10.1136/annrheumdis-2020-217176 on 6 April 2020. Downloaded from EPIDEMIOLOGICAL SCIENCE Associations of regular glucosamine use with all- cause and cause- specific mortality: a large prospective cohort study Zhi- Hao Li,1 Xiang Gao,2 Vincent CH Chung,3 Wen- Fang Zhong,1 Qi Fu,3 Yue- Bin Lv,4 Zheng- He Wang,1 Dong Shen,1 Xi- Ru Zhang,1 Pei-Dong Zhang,1 Fu- Rong Li,1 Qing- Mei Huang,1 Qing Chen,1 Wei- Qi Song,1 Xian- Bo Wu,1 Xiao-Ming Shi,4 Virginia Byers Kraus,5 Xingfen Yang,6 Chen Mao 1 Handling editor Josef S ABSTRact Key messages Smolen Objectives To evaluate the associations of regular glucosamine use with all-cause and cause-specific ► Additional material is What is already known about this subject? published online only. To view mortality in a large prospective cohort. ► Although several epidemiological investigations please visit the journal online Methods This population- based prospective cohort indicated that glucosamine use might play a (http:// dx. doi. org/ 10. 1136/ study included 495 077 women and men (mean (SD) role in prevention of cancer, cardiovascular annrheumdis- 2020- 217176). age, 56.6 (8.1) years) from the UK Biobank study. disease (CVD), and other diseases, only a Participants were recruited from 2006 to 2010 and For numbered affiliations see few studies have evaluated the associations were followed up through 2018. We evaluated all-cause end of article. between glucosamine use and mortality mortality and mortality due to cardiovascular disease outcomes, especially for cause- specific Correspondence to (CVD), cancer, respiratory and digestive disease. -

Osteoarthritis (OA) Is a Pain
R OBE 201 CT 1 O Managing Osteoarthritis With Nutritional Supplements Containing Glucosamine, Chondroitin Sulfate, and Avocado/Soybean Unsaponifiables steoarthritis (OA) is a pain- successfully recommending OJHSs treatment algorithm exists for man- ful, progressive degeneration containing a combination of glu- aging patients with OA, acetamin- O of the joint characterized by cosamine, chondroitin sulfate (CS), ophen is a first-line drug recom- progressive cartilage loss, subchon- and avocado/soybean unsaponifi- mended to help control pain and dral bone remodeling, formation of ables (ASU) for the management increase mobility. Acetaminophen periarticular osteophytes, and mild of OA. It will briefly review the is associated with both hepatic and to moderate synovitis accompanied available pharmacokinetics/phar- renal damage, particularly in patients by an increase in pro-inflammatory macodynamics and safety profile who exceed the recommended daily cytokines, chemokines, and a vari- of OJHSs containing a mixture of dose or use it in combination with ety of inflammatory mediators.1 glucosamine, CS, and ASU, outline moderate amounts of alcohol. 6 There is no cure for OA; therefore, strategies to identify appropriate Worsening pain, disease progression, once an individual is diagnosed, patients for OJHSs containing these and inefficacy of acetaminophen managing OA becomes a lifelong ingredients, and provide web-based often necessitate initiating prescrip- process. Although a physician ini- resources (TABLE 1) that will assist tion NSAIDs (such as COX-2 tially diagnoses OA, pharmacists are pharmacists in educating and coun- inhibitors), which carry the risk of the most accessible health care pro- seling patients. gastrointestinal effects (e.g., gas- fessional and play an integral role tropathy), renal toxicities, and seri- in managing each patient’s joint CURRENT RECOMMENDATIONS ous cardiovascular events. -

What Are the Acute Treatments for Migraine and How Are They Used?
2. Acute Treatment CQ II-2-1 What are the acute treatments for migraine and how are they used? Recommendation The mainstay of acute treatment for migraine is pharmacotherapy. The drugs used include (1) acetaminophen, (2) non-steroidal anti-inflammatory drugs (NSAIDs), (3) ergotamines, (4) triptans and (5) antiemetics. Stratified treatment according to the severity of migraine is recommended: use NSAIDs such as aspirin and naproxen for mild to moderate headache, and use triptans for moderate to severe headache, or even mild to moderate headache when NSAIDs were ineffective in the past. It is necessary to give guidance and cautions to patients having acute attacks, and explain the methods of using medications (timing, dose, frequency of use) and medication use during pregnancy and breast-feeding. Grade A Background and Objective The objective of acute treatment is to resolve the migraine attack completely and rapidly and restore the patient’s normal functions. An ideal treatment should have the following characteristics: (1) resolves pain and associated symptoms rapidly; (2) is consistently effective; (3) no recurrence; (4) no need for additional use of medication; (5) no adverse effects; (6) can be administered by the patients themselves; and (7) low cost. Literature was searched to identify acute treatments that satisfy the above conditions. Comments and Evidence The acute treatment drugs for migraine generally include (1) acetaminophens, (2) non-steroidal anti-inflammatory drugs (NSAIDs), (3) ergotamines, (4) triptans, and (5) antiemetics. For severe migraines including status migrainosus and migraine attacks refractory to treatment, (6) anesthetics, and (7) corticosteroids (dexamethasone) are used (Tables 1 and 2).1)-9) There are two approaches to the selection and sequencing of these medications: “step care” and “stratified care”. -

Native State Stabilization by Nsaids Inhibits Transthyretin Amyloidogenesis from the Most Common Familial Disease Variants
Laboratory Investigation (2004) 84, 545–552 & 2004 USCAP, Inc All rights reserved 0023-6837/04 $25.00 www.laboratoryinvestigation.org Native state stabilization by NSAIDs inhibits transthyretin amyloidogenesis from the most common familial disease variants Sean R Miller, Yoshiki Sekijima and Jeffery W Kelly Department of Chemistry and The Skaggs Institute of Chemical Biology, The Scripps Research Institute, La Jolla, CA 92037, USA Transthyretin (TTR) tetramer dissociation and misfolding affords a monomeric amyloidogenic intermediate that misassembles into aggregates including amyloid fibrils. Amyloidogenesis of wild-type (WT) TTR causes senile systemic amyloidosis (SSA), whereas fibril formation from one of the more than 80 TTR variants leads to familial amyloidosis, typically with earlier onset than SSA. Several nonsteroidal anti-inflammatory drugs (NSAIDs) stabilize the native tetramer, strongly inhibiting TTR amyloid fibril formation in vitro. Structure-based designed NSAID analogs are even more potent amyloid inhibitors. The effectiveness of several NSAIDs, including diclofenac, diflunisal, and flufenamic acid, as well as the diclofenac analog, 2–[(3,5-dichlorophenyl) amino] benzoic acid (inhibitor 1), has been demonstrated against WT TTR amyloidogenesis. Herein, the efficacy of these compounds at preventing acid-induced fibril formation and urea-induced tetramer dissociation of the most common disease-associated TTR variants (V30M, V122I, T60A, L58H, and I84S) was evaluated. Homotetramers of these variants were employed for the studies within, realizing that the tetramers in compound heterozygote patients are normally composed of a mixture of WT and variant subunits. The most common familial TTR variants were stabilized substantially by flufenamic acid and inhibitor 1, and to a lesser extent by diflunisal, against acid-mediated fibril formation and chaotrope denaturation, suggesting that this chemotherapeutic option is viable for patients with familial transthyretin amyloidosis. -

Efficacy and Safety of Celecoxib
ORIGINAL PAPER Nagoya J. Med. Sci. 77. 81 ~ 93, 2015 EFFICACY AND SAFETY OF CELECOXIB COMPARED WITH PLACEBO AND ETODOLAC FOR ACUTE POSTOPERATIVE PAIN: A MULTICENTER, DOUBLE-BLIND, RANDOMIZED, PARALLEL-GROUP, CONTROLLED TRIAL NAOKI ISHIGURO1, MD, PhD; AKIO HANAOKA2, MS; TOSHIYUKI OKADA2, MS; and MASANORI ITO3, PhD 1Department of Orthopedic Surgery, Nagoya University Graduate School of Medicine, Nagoya, Japan 2Clinical Development 1, Astellas Pharma Inc., Tokyo, Japan 3Global Data Science, Astellas Pharma Global Development Inc., Northbrook, IL, US ABSTRACT Celecoxib is a nonsteroidal anti-inflammatory drug (selective cyclooxygenase-2 inhibitor) that is widely used. The efficacy and safety of celecoxib for treatment of acute postoperative pain were evaluated in Japanese patients. The objective was to assess whether celecoxib showed superiority over placebo treatment and non-inferiority versus etodolac (another selective cyclooxygenase-2 inhibitor) that has been widely used for the management of acute pain. A multicenter, double-blind, randomized, parallel-group, controlled study was performed, in which 616 patients with postoperative pain received celecoxib, etodolac, or placebo. Their impressions of study drug efficacy (overall assessment) and pain intensity were evaluated. Based on each patient’s overall assessment of pain, the efficacy rate was 63.7% in the placebo group, 76.2% in the celecoxib group, and 68.0% in the etodolac group, with these results demonstrating superiority of celecoxib to placebo and noninferiority versus etodolac. The efficacy rate was significantly higher in the celecoxib group than in the etodolac group. There were no adverse events specific to celecoxib, and the safety of celecoxib was similar to that of placebo. Celecoxib was superior to etodolac for controlling acute postoperative pain. -

New Restrictions on Celecoxib (Celebrex) Use and the Withdrawal of Valdecoxib (Bextra)
Early release, published at www.cmaj.ca on April 15, 2005. Subject to revision. HEALTH AND DRUG ALERTS P RACTICE New restrictions on celecoxib (Celebrex) use and the withdrawal of valdecoxib (Bextra) Early release, published at www.cmaj.ca on Apr. 15, 2005. Subject to revision. Reason for posting: Coxibs, v. 0.5%; risk ratio 3.7, 95% CI the class of NSAIDs that selec- 1.0–13.5).2 Amid concerns about Table 1: The degree of inhibition of COX-2 relative tively inhibit cyclooxygenase 2 reports of severe cutaneous reac- to COX-1 for various NSAIDs (COX-2), were designed to re- tions (Stevens–Johnson syn- NSAID type COX-2 selectivity* duce joint pain and inflamma- drome, erythema multiforme, tion without causing the gastric toxic epidermal necrolysis) COX-2 selective inhibitors epithelial adverse effects typical among patients taking valde- Rofecoxib 80 of nonselective NSAIDs. Rofe- coxib,5 the drug was removed Etodolac 23 coxib (Vioxx) was withdrawn from the market. Meloxicam 11 from the market in September Celecoxib 9 2004 over concerns about car- What to do: COX-2 inhibitors Nonselective NSAIDs diovascular adverse effects, and appear to increase the risk of car- Diclofenac 4 key safety trials involving cele- diovascular adverse events in a Sulindac 3 coxib (Celebrex)1 and valdecoxib dose-related fashion, and all pa- Piroxicam 2 (Bextra)2 have recently been tients should be informed of this. Ibuprofen 0.4 published. Health Canada now Calculating the patient’s baseline Naproxen 0.3 recommends new restrictions on risk of cardiovascular disease Indomethacin 0.2 celecoxib use, and valdecoxib (e.g., with Framingham risk cal- Ketorolac 0.003 has been taken off the market.3 culators) may be wise, and cele- Note: COX = cyclooxygenase. -

Spondyloarthritis: NMA on Pain Outcome RR111467
NICE CGTSU Spondyloarthritis: NMA on Pain Outcome RR111467 Spondyloarthritis: NMA on pain outcome CGTSU, Bristol (Edna Keeney and Sofia Dias) The purpose of this analysis was to estimate the comparative effectiveness of the following pharmacological interventions for management of pain associated with axial spondyloarthritis: 1. Indomethacin (Reference) 2. Diclofenac 3. Sulindac 4. Fenoprofen 5. Ketoprofen 6. Flurbiprofen 7. Tenoxicam 8. Piroxicam 9. Celecoxib 200mg 10. Celecoxib 400mg 11. Aceclofenac 12. Naproxen 13. Enteric coated Naproxen 14. Etoricoxib 15. Tolfenamic acid 16. Meloxicam 15mg 17. Placebo 23 studies were included in the analyses. The network diagram is shown in Figure 1. Edna Keeney 1 13/10/2015 NICE CGTSU Spondyloarthritis: NMA on Pain Outcome RR111467 Figure 1. Network Diagram for pain outcome Pain Indomethacin Placebo Diclofenac Meloxicam 15mg Sulindac Tolfenamic acid Fenoprofen Etoricoxib Ketoprofen Enteric coated naproxen Flurbiprofen Naproxen Tenoxicam Piroxicam Aceclofenac Celecoxib 400mg Celecoxib 200mg METHODS In order to take all trial information into consideration, Mixed Treatment Comparison meta-analytic techniques, also termed Network meta-analysis (NMA), were employed. NMA is a generalization of standard pairwise meta-analysis for A versus B trials, to data structures that include, for example, A versus B, B versus C, and A versus C trials.1-3 A basic assumption of NMA methods is that direct and indirect evidence estimate the same parameter, that is, the relative effect between A and B measured directly -

CELEBREX™ (Celecoxib Capsules)
01/05/99 4:16 PM draft label 1 CELEBREX™ 2 (celecoxib capsules) 3 4 5 DESCRIPTION 6 7 CELEBREX (celecoxib) is chemically designated as 4-[5-(4-methylphenyl)-3- 8 (trifluoromethyl)-1H-pyrazol-1-yl] benzenesulfonamide and is a diaryl substituted 9 pyrazole. It has the following chemical structure: 10 O NH2 S O N N CF3 11 CH3 12 13 14 The empirical formula for celecoxib is C17H14F3N3O2S, and the molecular weight is 381.38. 15 16 CELEBREX oral capsules contain 100 mg and 200 mg of celecoxib. 17 18 The inactive ingredients in CELEBREX capsules include: croscarmellose sodium, edible 19 inks, gelatin, lactose monohydrate, magnesium stearate, povidone, sodium lauryl sulfate 20 and titanium dioxide. 21 22 CLINICAL PHARMACOLOGY 23 24 Mechanism of Action 25 CELEBREX is a nonsteroidal anti-inflammatory drug that exhibits anti-inflammatory, 26 analgesic, and antipyretic activities in animal models. The mechanism of action of 27 CELEBREX is believed to be due to inhibition of prostaglandin synthesis, primarily via 28 inhibition of cyclooxygenase-2 (COX-2), and at therapeutic concentrations in humans, 29 CELEBREX does not inhibit the cyclooxygenase-1 (COX-1) isoenzyme. 30 31 Pharmacokinetics 32 33 Absorption 34 Peak plasma levels of celecoxib occur approximately 3 hrs after an oral dose. Both peak 35 plasma levels (Cmax) and area under the curve (AUC) are roughly dose proportional 36 across the clinical dose range of 100-200 mg studied. At higher doses, under fasting 37 conditions, there is a less than proportional increase in Cmax and AUC which is thought 38 to be due to the low solubility of the drug in aqueous media. -

New Zealand Data Sheet 1
New Zealand Data Sheet 1. PRODUCT NAME NAXEN® 250 mg tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each NAXEN 250 mg tablet contains 250 mg of Naproxen For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM NAXEN 250 mg tablets are yellow, biconvex, round tablet of 11 mm diameter with one face engraved NX250 and having a bisecting score. The score line is only to facilitate breaking for ease of swallowing and not to divide into equal doses. 4. CLINICAL PARTICULARS 4.1. Therapeutic indications NAXEN is indicated in adults for the relief of symptoms associated with rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, tendonitis and bursitis, acute gout and primary dysmenorrhoea. NAXEN is indicated in children for juvenile arthritis. 4.2. Dose and method of administration After assessing the risk/benefit ratio in each individual patient, the lowest effective dose for the shortest possible duration should be used (see Section 4.4). During long-term administration the dose of naproxen may be adjusted up or down depending on the clinical response of the patient. A lower daily dose may suffice for long-term administration. In patients who tolerate lower doses well, the dose may be increased to 1000 mg per day when a higher level of anti-inflammatory/analgesic 1 | P a g e activity is required. When treating patients with naproxen 1000 mg/day, the physician should observe sufficient increased clinical benefit to offset the potential increased risk. Dose Adults For rheumatoid arthritis, osteoarthritis and ankylosing spondylitis Initial therapy: The usual dose is 500-1000 mg per day taken in two doses at 12 hour intervals.