The Escape of Planetary Atmospheres
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
The Geology of the Rocky Bodies Inside Enceladus, Europa, Titan, and Ganymede
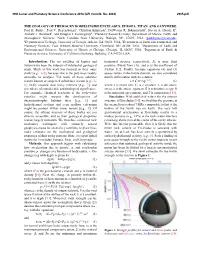
49th Lunar and Planetary Science Conference 2018 (LPI Contrib. No. 2083) 2905.pdf THE GEOLOGY OF THE ROCKY BODIES INSIDE ENCELADUS, EUROPA, TITAN, AND GANYMEDE. Paul K. Byrne1, Paul V. Regensburger1, Christian Klimczak2, DelWayne R. Bohnenstiehl1, Steven A. Hauck, II3, Andrew J. Dombard4, and Douglas J. Hemingway5, 1Planetary Research Group, Department of Marine, Earth, and Atmospheric Sciences, North Carolina State University, Raleigh, NC 27695, USA ([email protected]), 2Department of Geology, University of Georgia, Athens, GA 30602, USA, 3Department of Earth, Environmental, and Planetary Sciences, Case Western Reserve University, Cleveland, OH 44106, USA, 4Department of Earth and Environmental Sciences, University of Illinois at Chicago, Chicago, IL 60607, USA, 5Department of Earth & Planetary Science, University of California Berkeley, Berkeley, CA 94720, USA. Introduction: The icy satellites of Jupiter and horizontal stresses, respectively, Pp is pore fluid Saturn have been the subjects of substantial geological pressure (found from (3)), and μ is the coefficient of study. Much of this work has focused on their outer friction [12]. Finally, because equations (4) and (5) shells [e.g., 1–3], because that is the part most readily assess failure in the brittle domain, we also considered amenable to analysis. Yet many of these satellites ductile deformation with the relation n –E/RT feature known or suspected subsurface oceans [e.g., 4– ε̇ = C1σ exp , (6) 6], likely situated atop rocky interiors [e.g., 7], and where ε̇ is strain rate, C1 is a constant, σ is deviatoric several are of considerable astrobiological significance. stress, n is the stress exponent, E is activation energy, R For example, chemical reactions at the rock–water is the universal gas constant, and T is temperature [13]. -

Astronomy 330 HW 2 Presentations Outline
Astronomy 330 HW 2 •! Stanley Swat This class (Lecture 12): http://www.ufohowto.com/ Life in the Solar System •! Lucas Guthrie Next Class: http://www.crystalinks.com/abduction.html Life in the Solar System HW 5 is due Wednesday Music: We Are All Made of Stars– Moby Presentations Outline •! Daniel Borup •! Life on Venus? Futurama •! Life on Mars? Life in the Solar System? Earth – Venus comparison •! We want to examine in more detail the backyard of humans. •! What we find may change our estimates of ne. Radius 0.95 Earth Surface gravity 0.91 Earth Venus is the hottest Mass 0.81 Earth planet, the closest in Distance from Sun 0.72 AU size to Earth, the closest Average Temp 475 C in distance to Earth, and Year 224.7 Earth days the planet with the Length of Day 116.8 Earth days longest day. Atmosphere 96% CO2 What We Used to Think Turns Out that Venus is Hell Venus must be hotter, as it is closer the Sun, but the cloud •! The surface is hot enough to melt lead cover must reflect back a large amount of the heat. •! There is a runaway greenhouse effect •! There is almost no water In 1918, a Swedish chemist and Nobel laureate concluded: •! There is sulfuric acid rain •! Everything on Venus is dripping wet. •! Most of the surface is no doubt covered with swamps. •! Not a place to visit for Spring Break. •! The constantly uniform climatic conditions result in an entire absence of adaptation to changing exterior conditions. •! Only low forms of life are therefore represented, mostly no doubt, belonging to the vegetable kingdom; and the organisms are nearly of the same kind all over the planet. -

Exomoon Habitability Constrained by Illumination and Tidal Heating
submitted to Astrobiology: April 6, 2012 accepted by Astrobiology: September 8, 2012 published in Astrobiology: January 24, 2013 this updated draft: October 30, 2013 doi:10.1089/ast.2012.0859 Exomoon habitability constrained by illumination and tidal heating René HellerI , Rory BarnesII,III I Leibniz-Institute for Astrophysics Potsdam (AIP), An der Sternwarte 16, 14482 Potsdam, Germany, [email protected] II Astronomy Department, University of Washington, Box 951580, Seattle, WA 98195, [email protected] III NASA Astrobiology Institute – Virtual Planetary Laboratory Lead Team, USA Abstract The detection of moons orbiting extrasolar planets (“exomoons”) has now become feasible. Once they are discovered in the circumstellar habitable zone, questions about their habitability will emerge. Exomoons are likely to be tidally locked to their planet and hence experience days much shorter than their orbital period around the star and have seasons, all of which works in favor of habitability. These satellites can receive more illumination per area than their host planets, as the planet reflects stellar light and emits thermal photons. On the contrary, eclipses can significantly alter local climates on exomoons by reducing stellar illumination. In addition to radiative heating, tidal heating can be very large on exomoons, possibly even large enough for sterilization. We identify combinations of physical and orbital parameters for which radiative and tidal heating are strong enough to trigger a runaway greenhouse. By analogy with the circumstellar habitable zone, these constraints define a circumplanetary “habitable edge”. We apply our model to hypothetical moons around the recently discovered exoplanet Kepler-22b and the giant planet candidate KOI211.01 and describe, for the first time, the orbits of habitable exomoons. -

Modeling the Formation of the Earth's Atmosphere by Hydrodynamic
Origin of the Earth and Moon Conference 4102.pdf MODELING THE FORMATION OF THE EARTH’S ATMOSPHERE BY HYDRODYNAMIC ESCAPE AND PLANETARY OUTGASSING. R. O. Pepin, School of Physics and Astronomy, University of Minnesota, Minneapolis MN 55455, USA. Accretion of lunar- to Mars-sized terrestrial Models of this kind have had some success in planet embryos is believed to have occurred on accounting for the details of terrestrial noble gas timescales of »105 years in the presence of nebular mass distributions [3,6]. However, they are not with- gas [1,2]. Mechanisms for trapping nebular (“solar”) out problems. “Solar” isotopic distributions in the noble gases in these embryos include occlusion of initial terrestrial reservoirs are taken to be those ambient gases in the planetesimals accreted to form measured in the solar wind. Although current esti- them, and, probably more important, efficient ad- mates for the isotopic compositions of solar-wind Ne, sorption of gravitationally condensed nebular gases Ar, and Kr are compatible with those required in the on embryo surfaces once they had grown to modeling for Earth’s primordial noble gas invento- ~Mercury size [3]. During the following ~100–200 ries, this assumption that the wind correctly repre- m.y. of growth through the “giant-impact” stage sents the composition of the nebular source supply- [1,4] to a fully assembled planet, a coaccreting pri- ing these gases to the early Earth is not strictly valid mordial atmosphere is likely to develop by impact- for Xe. Generating the isotope ratios of terrestrial degassing of colliding embryos and inward-scattered nonradiogenic Xe by fractionation in hydrodynamic icy planetesimals, and by gravitational capture of escape requires an initial composition called U-Xe, nebular gases if the gas phase of the nebula had not which appears to be isotopically identical to meas- yet fully dissipated. -

Conceptual Design for a Sputter-Type Negative Ion Source Based
MOPO-03 Proceedings of ECRIS08, Chicago, IL USA CONCEPTUAL DESIGN OF A SPUTTER-TYPE NEGATIVE ION SOURCE BASED ON ELECTRON CYCLOTRON RESONANCE PLASMA HEATING O. Tarvainen and S. Kurennoy, Los Alamos National Laboratory, Los Alamos, NM 87545, U.S.A. Abstract PHYSICS ASPECTS OF H- ION BEAM A design for a negative ion source based on electron PRODUCTION cyclotron resonance plasma heating and ionization by Modern H- ion sources are based on two important ion surface sputtering is presented. The plasma chamber of formation processes, the volume [3] and the surface [4] the source is an rf-cavity designed for TE111 eigenmode at production. The relative importance of these processes 2.45 GHz. The desired mode is excited with a loop depends on the detailed design of the ion source. antenna. The ionization process takes place on a cesiated The volume production of H- is generally accepted to surface of a biased converter electrode (cathode). The ion be due to dissociative attachment (DA) of low energy beam is further “self-extracted” through the plasma electrons to rovibrationally (ν,,) excited molecules. Two region. The magnetic field of the source is optimized for electron populations are needed in order to optimize the both, plasma generation by electron cyclotron resonance volume production process: hot electrons (few eV) heating, and beam extraction. The source can be used for creating the excited molecular states i.e. ehot + H2 Æ e + a production of a variety of negative ions ranging from v’’ H2 (v’’ > 5) and cold electrons (less than 1 eV) hydrogen to heavy ions. -

Sputter Coating Technical Brief
Sputter Coating Technical Brief Document Number TB-SPUTTER Issue 2 (01/02) Introduction HP000107 Quorum Technologies Ltd main sales office: South Stour Avenue Ashford Kent U.K. Tel: ++44(0) 1233 646332 TN23 7RS Fax: ++44(0) 1233 640744 Email: [email protected] http:///www.quorumtech.com For further information regarding any of the other products designed and manufactured by Quorum Technologies, contact your local representative or directly to Quorum Technologies at the address above. Carbon and sputter coaters Plasma reactor for ashing and etching High vacuum bench top evaporators Cryo-SEM preparation systems Critical point dryers Freeze dryers for electron microscopy Service and Spares Disclaimer The components and packages described in this document are mutually compatible and guaranteed to meet or exceed the published performance specifications. No performance guarantees, however, can be given in circumstances where these component packages are used in conjunction with equipment supplied by companies other than Quorum Technologies. Quorum Technologies Limited, Company No. 04273003 Registered office: Unit 19, Charlwoods Road, East Grinstead, West Sussex, RH19 2HL, UK TB-SPUTTER Contents Contents Chapter 1 - Introduction .......................................................................................... 4 Chapter 2 - Gaseous Condition .............................................................................. 5 Chapter 3 - Glow Discharge ................................................................................... -

Lecture 23: Jupiter Solar System Jupiter's Orbit
Lecture 23: Jupiter Solar System Jupiter’s Orbit •The semi-major axis of Jupiter’s orbit is a = 5.2 AU Jupiter Sun a •Kepler’s third law relates the semi-major axis to the orbital period 1 Jupiter’s Orbit •Kepler’s third law relates the semi-major axis a to the orbital period P 2 3 ⎛ P ⎞ = ⎛ a ⎞ ⎜ ⎟ ⎜ ⎟ ⎝ years⎠ ⎝ AU ⎠ •Solving for the period P yields 3/ 2 ⎛ P ⎞ = ⎛ a ⎞ ⎜ ⎟ ⎜ ⎟ ⎝ years ⎠ ⎝ AU ⎠ •Since a = 5.2 AU for Jupiter, we obtain P = 11.9 Earth years •Jupiter’s orbit has eccentricity e = 0.048 Jupiter’s Orbit •The distance from the Sun varies by about 10% during an orbit Dperihelion = 4.95 AU Daphelion = 5.45 AU •Like Mars, Jupiter is easiest to observe during favorable opposition •At this time, the Earth-Jupiter distance is only about 3.95 AU Earth Jupiter Jupiter Sun (perihelion) (aphelion) •Jupiter appears full during favorable opposition Bulk Properties of Jupiter •Jupiter is the largest planet in the solar system •We have for the radius and mass of Jupiter Rjupiter = 71,400 km = 11.2 Rearth 30 Mjupiter = 1.9 x 10 g = 318 Mearth •The volume of Jupiter is therefore given by 4 V = π R 3 jupiter 3 jupiter 30 3 •Hence Vjupiter = 1.5 x 10 cm 2 Bulk Properties of Jupiter •The average density of Jupiter is therefore M × 30 ρ = jupiter = 1.9 10 g jupiter × 30 3 Vjupiter 1.5 10 cm •We obtain ρ = −3 jupiter 1.24 g cm •This is much less than the average density of the Earth •Hence there is little if any iron and no dense core inside Jupiter Bulk Properties of Jupiter •The average density of the Earth is equal to −3 ρ⊕ = 6 g cm •The density -

Water on Venus: Implications of Theearly Hydrodynamic Escape
EPSC Abstracts Vol. 5, EPSC2010-288, 2010 European Planetary Science Congress 2010 c Author(s) 2010 Water on Venus: Implications of theEarly Hydrodynamic Escape C. Gillmann (1), E. Chassefière (2) and P. Lognonné (1) (1) Institut de Physique du Globe (IPGP), Paris, France, ([email protected]) (2) CNRS/UPS UMR 8148 IDES Interactions et Dynamique des Environnements de Surface, Paris, France Abstract toward a common origin for those three atmospheres and a usual theory is that these atmospheres are In order to study the evolution of the primitive secondary, created by the degassing of volatiles from atmosphere of Venus, we developed a time the bodies that constituted the early planet. The dependent model of hydrogen hydrodynamic escape atmosphere of Venus could then represent a primitive state of the evolution of terrestrial. Moreover, Mars powered by solar EUV (Extreme UV) flux and solar and the Earth possess reservoirs of water at present- wind, and accounting for oxygen frictional escape day whereas Venus seems to be dry. The early We study specifically the isotopic fractionation of evolution of terrestrial planets and the effects of noble gases resulting from hydrodynamic escape. hydrodynamic escape might explain this observation The fractionation’s primary cause is the effect of by the removal of most of the initial water on Venus. diffusive/gravitational separation between the homopause and the base of the escape. Heavy noble 2. Results and Scenario gases such as Kr and Xe are not fractionated. Ar is only marginally fractionated whereas Ne is We study the evolution of the primitive atmosphere moderately fractionated. of Venus and investigate the possibility of an early We also take into account oxygen dragged off habitable Venus with a possible liquid water ocean on along with hydrogen by hydrodynamic process. -

Prebiotic Chemistry, Origin, and Early Evolution of Life
Say what you are going to say, say it, say what you said Guiding theory Richard Feynman Polyelectrolytes with uniform structure are universal for Darwinism A specific hypothesis to provide context The polyelectrolyte that supported Earth’s first Darwinism was RNA Focus on paradoxes to constrain human self-deception "Settled science" says that RNA is impossible to form prebiotically Strategies for paradox resolution Mineral-guided processes allow RNA to form nonetheless Natural history context The needed chemistry-mineral combination was transient on Earth Your reward A relatively simple path to form RNA prebiotically A relatively narrow date when life on Earth originated prebiotically A clear statement of the next round of paradoxes Elisa Biondi, Hyo-Joong Kim, Daniel Hutter, Clemens Richert, Stephen Mojzsis, Ramon Brasser, Dustin Trail, Kevin Zahnle, David Catling, Rob Lavinsky Say what you are going to say, say it, say what you said Guiding theory Richard Feynman Polyelectrolytes with uniform structure are universal for Darwinism A specific hypothesis to provide context The polyelectrolyte that supported Earth’s first Darwinism was RNA Focus on paradoxes to constrain human self-deception "Settled science" says that RNA is impossible to form prebiotically Strategies for paradox resolution Mineral-guided processes allow RNA to form nonetheless Natural history context The needed chemistry-mineral combination was transient on Earth Your reward A relatively simple path to form RNA prebiotically A relatively narrow date when life on Earth originated prebiotically A clear statement of the next round of paradoxes Elisa Biondi, Hyo-Joong Kim, Daniel Hutter, Clemens Richert, Stephen Mojzsis, Ramon Brasser, Dustin Trail, Kevin Zahnle, David Catling, Rob Lavinsky What does a repeating backbone charge do for informational molecule? O 1. -

Snowball Earth: a Thin-Ice Solution with Flowing Sea Glaciers’’ by David Pollard and James F
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 111, C09016, doi:10.1029/2005JC003411, 2006 Click Here for Full Article Comment on ‘‘Snowball Earth: A thin-ice solution with flowing sea glaciers’’ by David Pollard and James F. Kasting Stephen G. Warren1,2 and Richard E. Brandt1 Received 22 November 2005; revised 22 May 2006; accepted 9 June 2006; published 14 September 2006. Citation: Warren, S. G., and R. E. Brandt (2006), Comment on ‘‘Snowball Earth: A thin-ice solution with flowing sea glaciers’’ by David Pollard and James F. Kasting, J. Geophys. Res., 111, C09016, doi:10.1029/2005JC003411. 1. Introduction 2. Choices of Model Variables That Favor [1] Pollard and Kasting [2005] (hereinafter referred to as Thin Ice PK) have coupled an energy-balance climate model to an 2.1. Albedo of Cold Glacier Ice ice-shelf flow model, to investigate the Snowball Earth [2] As sea glaciers flowed equatorward into the tropical episodes of the Neoproterozoic, 600–800 million years region of net sublimation, their surface snow and subsurface ago, when the ocean apparently froze all the way to the firn would sublimate away, exposing bare glacier ice to equator [Hoffman and Schrag, 2002]. PK’s particular con- the atmosphere and solar radiation. This ice would be cern was to investigate the possibility that over a wide freshwater (meteoric) ice, which originated from compres- equatorial band where sublimation exceeded snowfall, the sion of snow, so it would contain numerous bubbles, giving bare ice may have been thin enough to permit transmission a high albedo. The albedo of cold (nonmelting) glacier ice of sunlight to the water below, providing an extensive exposed by sublimation (Antarctic ‘‘blue ice’’) has been refugium for the photosynthetic eukaryotes that survived measured as 0.55–0.65 in four experiments in the Atlantic the Snowball events. -

Thermal Structure and Composition of Jupiter's Great Red Spot from High-Resolution Thermal Imaging
Thermal Structure and Composition of Jupiter’s Great Red Spot from High-Resolution Thermal Imaging Leigh N. Fletchera,b, G. S. Ortona, O. Mousisc,d, P. Yanamandra-Fishera, P. D. Parrishe,a, P. G. J. Irwinb, B. M. Fishera, L. Vanzif, T. Fujiyoshig, T. Fuseg, A.A. Simon-Millerh, E. Edkinsi, T.L. Haywardj, J. De Buizerk aJet Propulsion Laboratory, California Institute of Technology, 4800 Oak Grove Drive, Pasadena, CA, 91109, USA bAtmospheric, Oceanic and Planetary Physics, Department of Physics, University of Oxford, Clarendon Laboratory, Parks Road, Oxford, OX1 3PU, UK cInstitut UTINAM, CNRS-UMR 6213, Observatoire de Besan¸con, Universit´ede Franche-Comt´e, Besan¸con, France dLunar and Planetary Laboratory, University of Arizona, Tucson, AZ, USA eSchool of GeoScience, University of Edinburgh, Crew Building, King’s Buildings, Edinburgh, EH9 3JN, UK fPontificia Universidad Catolica de Chile, Department of Electrical Engineering, Av. Vicuna Makenna 4860, Santiago, Chile. gSubaru Telescope, National Astronomical Observatory of Japan, National Institutes of Natural Sciences, 650 North A’ohoku Place, Hilo, Hawaii 96720, USA hNASA/Goddard Spaceflight Center, Greenbelt, Maryland, 20771, USA iUniversity of California, Santa Barbara, Santa Barbara, CA 93106, USA jGemini Observatory, Southern Operations Center, c/o AURA, Casilla 603, La Serena, Chile. kSOFIA - USRA, NASA Ames Research Center, Moffet Field, CA 94035, USA. Abstract Thermal-IR imaging from space-borne and ground-based observatories was used to investigate the temperature, composition and aerosol structure of Jupiter’s Great Red Spot (GRS) and its temporal variability between 1995-2008. An elliptical warm core, extending over 8◦ of longitude and 3◦ of latitude, was observed within the cold anticyclonic vortex at 21◦S. -

THE CHANTICLEER Jacksonville State University Jacksonville
Jacksonville State University JSU Digital Commons Chanticleer Historical Newspapers 1985-01-11 Chanticleer | Vol 32, Issue 15 Jacksonville State University Follow this and additional works at: https://digitalcommons.jsu.edu/lib_ac_chanty Recommended Citation Jacksonville State University, "Chanticleer | Vol 32, Issue 15" (1985). Chanticleer. 871. https://digitalcommons.jsu.edu/lib_ac_chanty/871 This Book is brought to you for free and open access by the Historical Newspapers at JSU Digital Commons. It has been accepted for inclusion in Chanticleer by an authorized administrator of JSU Digital Commons. For more information, please contact [email protected]. C Entertainment THE CHANTICLEER Jacksonville State university Jacksonville. Alabama Vol. &No. W January 11, 1985 Mild controversy erupts over - senate resolution By JAN DICKINSON A resolution calling for a faculty-wide vote of "no confidence" in Resident Theron Montgomery was presented to the Faculty Senate at its December 10 meeting, two days before Montgomery anriounced his resignation to the Board of Trustees. Now the sponsor of the resolution, Dr. Gene Blanton, of the English Department, says that he intends to withdraw the resolution at the January 14 meeting of the senate. 13lanton stated in the January 6 edition of the Anniston Star that, because of comments made by several members of the Board of bstees, the wssibility of dropping the resolution was strong. According to that article, trustee Paul Carpenter said, "It (the vote) wouid make absolutely no difference in my opinion. We have studied the entire situation, and we are satisfied with the decision." Another board member, Dwaine Luce, stated, "....the bard is responsible for running the school, and the faculty is responsible for iristructing the students." Echoing the sentiments of Carpenter, he added, "'I'he board has inade its decision and the board, in my pinion, will stand by its decision." According to Dr.