Red List of Baltic Breeding Birds
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Quantitative Composition and Granulometry of Aeolian Bedforms in Endeavour and Gale Craters Inferred from Visible Near-Infrared Spectra
45th Lunar and Planetary Science Conference (2014) 1431.pdf QUANTITATIVE COMPOSITION AND GRANULOMETRY OF AEOLIAN BEDFORMS IN ENDEAVOUR AND GALE CRATERS INFERRED FROM VISIBLE NEAR-INFRARED SPECTRA. Mathieu G.A. Lapotre1, Bethany L. Ehlmann1,2, Raymond E. Arvidson3. 1Division of Geological and Planetary Sciences, California Institute of Technology, Pasadena, CA, USA. 2Jet Propulsion Laboratory, California Institute of Technology, Pasadena, CA, USA, 3Department of Earth & Planetary Sciences, Washington University in St. Louis, MO, USA. Introduction: Modern Mars is a wind world. Its ing Spectrometer for Mars (CRISM) visible near- surface hosts a variety of aeolian features, such as line- infrared spectra (VISIR). The goal of this study is to ar, barchan and star dunes, ripples, granule ripples, compare inversions made from orbit to ground truth yardangs and ventifacts [1]. Even though active sand provided by instruments aboard Opportunity at En- transport was observed at the surface [2], it is not clear deavour Crater, Terra Meridiani and Curiosity in Gale whether all of the preserved aeolian bedforms are ac- crater. tive. In particular, transverse aeolian ridges have been We use Hapke’s bidirectional reflectance spectros- suggested to be remnant dunes that formed under past copy theory [6] to invert for optical constants of miner- climatic conditions [3]. als from laboratory spectra [e.g., 7, 8]. These are used Sand transport is largely controlled by the size and to compute single scattering albedos of mineral the density of the grains [4]. Moreover, dunes and rip- endmember components of varying grain sizes. We use ples form in unimodally distributed sand particles from an atmospheric radiative transfer approach, DISORT different instabilities, and the wavelengths of these [9], to correct the CRISM spectra for the effects of the different bedforms do not have the same dependence Martian atmosphere. -

Verordnung Über Das Befahren Der Bundeswasserstraßen in Nationalparken Und Naturschutzgebieten Im Bereich Der Küste Von Meckl
Ein Service des Bundesministeriums der Justiz und für Verbraucherschutz sowie des Bundesamts für Justiz ‒ www.gesetze-im-internet.de Verordnung über das Befahren der Bundeswasserstraßen in Nationalparken und Naturschutzgebieten im Bereich der Küste von Mecklenburg-Vorpommern (Befahrensregelungsverordnung Küstenbereich Mecklenburg-Vorpommern - NPBefVMVK) NPBefVMVK Ausfertigungsdatum: 24.06.1997 Vollzitat: "Befahrensregelungsverordnung Küstenbereich Mecklenburg-Vorpommern vom 24. Juni 1997 (BGBl. I S. 1542), die durch Artikel 30 der Verordnung vom 2. Juni 2016 (BGBl. I S. 1257) geändert worden ist" Stand: Geändert durch Art. 30 V v. 2.6.2016 I 1257 Fußnote (+++ Textnachweis ab: 10.7.1997 +++) Eingangsformel Auf Grund des § 5 Satz 3 des Bundeswasserstraßengesetzes in der Fassung der Bekanntmachung vom 23. August 1990 (BGBl. I S. 1818) verordnet das Bundesministerium für Verkehr im Einvernehmen mit dem Bundesministerium für Umwelt, Naturschutz und Reaktorsicherheit: § 1 (1) Zum Schutz der Tier- und Pflanzenwelt wird das Befahren der Bundeswasserstraßen mit Wasserfahrzeugen, Sportfahrzeugen oder Wassersportgeräten und der Betrieb von ferngesteuerten Schiffsmodellen in dem 1. Nationalpark "Vorpommersche Boddenlandschaft" gemäß der Verordnung über die Festsetzung des Nationalparks Vorpommersche Boddenlandschaft vom 12. September 1990 (GBl. Sonderdruck Nr. 1466), die nach Artikel 3 Kapitel XII Nr. 30 Buchstabe a der Vereinbarung zum Einigungsvertrag vom 18. September 1990 in Verbindung mit Artikel 1 des Gesetzes vom 23. September 1990 (BGBl. 1990 II S. 885, 1239) mit den dort genannten Maßgaben fortgilt, 2. Nationalpark "Jasmund" gemäß der Verordnung über die Festsetzung des Nationalparks Jasmund vom 12. September 1990 (GBl. Sonderdruck Nr. 1467), die nach Artikel 3 Kapitel XII Nr. 30 Buchstabe b der Vereinbarung zum Einigungsvertrag vom 18. September 1990 in Verbindung mit Artikel 1 des Gesetzes vom 23. -

Bilag 6 Bornholms Råstofindustri
Kulturarv BORNHOLMS RÅSTOFINDUSTRI ET NATIONALT INDUSTRIMINDE Kulturarvsstyrelsen, Bornholms Museum og Bornholms Regionskommune har indgået et samarbejde om at få beskrevet og afgrænset de bærende værdier i øens råstoflandskaber. Kortlægningen skal tydeliggøre landskabelige, kulturhistoriske og arkitektoniske sammenhænge, så de kan indgå som et aktiv i den fysiske planlægning og i den fremtidige udvikling af nogle af Danmarks vigtigste råstofindvindingsområder. DECEMBER 2011 0 Titel Bornholms Råstofindustri – Et nationalt industriminde Udgiver Bornholms Regionskommune i samarbejde med Kulturarvsstyrelsen og Bornholms Museum Udgivet December 2011 – i digital form til download Research, tekst og layout Konsulent Lis Jensen, landskabsarkitekt Kortgrundlag Kort‐ og Matrikelstyrelsen og Bornholms Regionskommune Indhold SAMMENFATNING ‐ BORNHOLMS RÅSTOFINDUSTRILANDSKAB .......................................................................................4 1 GRANITTENS BORNHOLM ......................................................................................................................16 2 SANDSTENENS BORNHOLM ...................................................................................................................42 3 LERETS BORNHOLM................................................................................................................................51 4 KULLETS BORNHOLM .............................................................................................................................77 5 CEMENTSTENENS OG -

Comparative Life History of the South Temperate Cape Penduline Tit (Anthoscopus Minutus) and North Temperate Remizidae Species
J Ornithol (2017) 158:569–577 DOI 10.1007/s10336-016-1417-4 ORIGINAL ARTICLE Comparative life history of the south temperate Cape Penduline Tit (Anthoscopus minutus) and north temperate Remizidae species 1,2 1 1 Penn Lloyd • Bernhard D. Frauenknecht • Morne´ A. du Plessis • Thomas E. Martin3 Received: 19 June 2016 / Revised: 22 October 2016 / Accepted: 14 November 2016 / Published online: 22 November 2016 Ó Dt. Ornithologen-Gesellschaft e.V. 2016 Abstract We studied the breeding biology of the south parental nestling care. Consequently, in comparison to the temperate Cape Penduline Tit (Anthoscopus minutus)in other two species, the Cape Penduline Tit exhibits greater order to compare its life history traits with those of related nest attentiveness during incubation, a similar per-nestling north temperate members of the family Remizidae, namely feeding rate and greater post-fledging survival. Its rela- the Eurasian Penduline Tit (Remiz pendulinus) and the tively large clutch size, high parental investment and Verdin (Auriparus flaviceps). We used this comparison to associated high adult mortality in a less seasonal environ- test key predictions of three hypotheses thought to explain ment are consistent with key predictions of the adult latitudinal variation in life histories among bird species— mortality hypothesis but not with key predictions of the the seasonality and food limitation hypothesis, nest pre- seasonality and food limitation hypothesis in explaining dation hypothesis and adult mortality hypothesis. Contrary life history variation among Remizidae species. These to the general pattern of smaller clutch size and lower adult results add to a growing body of evidence of the impor- mortality among south-temperate birds living in less sea- tance of age-specific mortality in shaping life history sonal environments, the Cape Penduline Tit has a clutch evolution. -

Mouse Breeding Colony Management 1. Mouse Reproduction A. General Mouse Information I. the Average Mouse Lives Approximately
Mouse Breeding Colony Management 1. Mouse Reproduction A. General Mouse Information i. The average mouse lives approximately 2.5 years; however, the reproductive life span of mice is significantly shorter at 7-8 months. ii. Most mice reach sexual maturity (males and females) at 4-7 weeks of age. Younger mice generally produce smaller litters and therefore are not typically mated until they reach 6-8 weeks, of age. Mice that have been housed alone or in same-sex pairs will usually not breed successfully if they are older than 6-8 months. iii. The mouse estrous cycle is 4-5 days in length. Mice cycle continuously throughout the year (non-seasonal breeders). Female mice are only receptive to males when they are in estrus. Mating typically occurs at night (lights off). Ovulation occurs 8-12 hours after the onset of estrous. iv. If fertilization occurs, fetuses can be palpated by day 14. v. Gestation in mice is typically 19-21 days (strain dependent). vi. Parturition in mice may last 1-3 hours and frequently occurs at night. Females will go into estrus within 24 hours of parturition and are sexually receptive during this time. vii. Litter size varies among strains, but averages 4-12 pups. Inbred mice tend to have smaller litters than outbred mice. viii. Mice are typically weaned at 21-28 days or at 10g of body weight. The Purdue Animal Care and Use Committee requires that mouse pups be weaned at 21 days unless PACUC approval is given on an approved animal use protocol. See Policy attached. -

Kevin Gill ‘11G
InSight: RIVIER ACADEMIC JOURNAL, VOLUME 14, NUMBER 1, FALL 2018 EXPLORING THE UNIVERSE: Meet Kevin Gill ‘11G Michelle Marrone (From Rivier Today, Fall 2018) From the comfort of his lab chair in sunny, southern California, Kevin Gill ’11G has a view into outer space. As a Science Data Software Engineer at NASA’s Jet Propulsion Laboratory (JPL), he spends his time planning and designing technology in support of environmental science and space exploration, as well as data visualization and planetary imaging. His recent work not only produced the first-ever close views of Saturn, but also contributed to NASA’s team winning an Emmy Award. Kevin earned his M.S. in Computer Science at Rivier and has been designing software to render the unique images he gathers ever since. He used an algorithm he developed during his program at Rivier to generate hypothetical images portraying Mars as a vibrant planet with oceans, an oxygen-rich atmosphere, and a green biosphere. The images went viral and within a week his work was featured on major media networks—Discovery News, Fox News, Universe Today, and the Huffington Post. His work captured NASA’s attention and paved the way for his career move. “Rivier taught me many of the algorithms and development practices I still use today at NASA,” says Kevin. “In fact, I can trace the lineage of code currently running on NASA systems directly to my final master’s project at the University.” The systems and tools he develops support a range of scientists specializing in the areas of climate, oceanography, asteroids, planetary science, and others. -

Selection of Processing Tomato Genotypes Resistant to Two Spotted Spider Mite
Scientific communication VALADARES, RN; MELO, RA; SARINHO, IVF; OLIVEIRA, NS; ROCHA, FAT; SILVA, JW; MENEZES, D. 2018. Genetic diversity in accessions of melon belonging to momordica group. Horticultura Brasileira 36: 253258. DOI: http://dx.doi.org/10.1590/S0102-053620180218 Genetic diversity in accessions of melon belonging to momordica group Ricardo N Valadares1; Roberto A Melo1; Isabel VF Sarinho1; Natália S Oliveira2; Fernando AT Rocha1; José W Silva1; Dimas Menezes1 1Universidade Federal Rural de Pernambuco (UFRPE), Recife-PE, Brazil; [email protected] (corresponding author); [email protected]; [email protected]; [email protected]; [email protected]; dimasmenezes@ superig.com.b; 2Universidade Federal de Lavras (UFLA), Lavras-MG, Brazil; [email protected] ABSTRACT RESUMO The genetic divergence of melon genotypes belonging to Divergência genética em acessos de melão do grupo momordica group, collected in five Brazilian States, was estimated, momordica and the relative contribution of the morphological characters was A divergência genética de genótipos de melão do grupo determined for the genetic variability. The experimental design was momordica foi estimada, coletados em cinco estados brasileiros, randomized blocks, with four replicates. We evaluated 19 accessions e determinada a contribuição relativa dos caracteres morfológicos of melon, momordica group, two accessions of cantaloupensis group avaliados para a variabilidade genética. Foi adotado o delineamento and two commercial cultivars of inodorus group. These genotypes de blocos casualizados com quatro repetições. Nesse estudo, were characterized by 42 morphological descriptors. The data were foram utilizados 19 acessos de melão do grupo momordica, dois submitted to Tocher and UPGMA grouping methods using the genetic acessos do grupo cantaloupensis e duas cultivares comerciais do dissimilarity matrix, using Mahalanobis’ distance. -

PUTTING LIFE on MARS: Using Computer Graphics to Render a Living Mars
InSight: RIVIER ACADEMIC JOURNAL, VOLUME 9, NUMBER 1, SPRING 2013 PUTTING LIFE ON MARS: Using Computer Graphics to Render a Living Mars Kevin M. Gill ‘11G* Senior Software Engineer, Thunderhead.com, Manchester, NH Keywords: Computer Graphics, Mars, Life, Planetary Science, OpenGL Abstract This article describes the software, algorithms & decisions that went into the development of the Living Mars image project. This includes topics related to computer graphics, software development, astronomy, & planetary science. The purpose of the project was to create a visualization of the planet Mars as could look with a living biosphere. This makes no distinction as to whether this biosphere would represent an ancient or future, possibly terraformed planet. 1 Background Mars, named for the Roman god of war. Ancient civilizations have forever associated the planet with fear, war, and destruction. It is the color of blood, and “one of a handful of planets visible to the naked eye, and the only one of marked color, so the planet demanded attention (Pyle, 2012).” Ever since man has noticed it, there have been dreams and visions of life on Mars, from Giovanni Schiaparelli and Percival Lowell describing channels and canals to Robert A. Heinlein’s science fiction. Lowell, in particular famous for fantastic writings of Mars, asked “are physical forces alone at work there, or has evolution begotten something more complex, something not unakin to what we know on Earth as life?” (Lowell, 1895) Even more recent discoveries by NASA’s Curiosity rover have found proof that liquid water once flowed billions of years ago positing an environment that could have served host to life (Brown, 2013). -
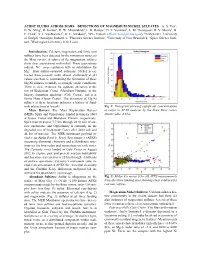
Acidic Fluids Across Mars: Detections of Magnesium-Nickel Sulfates
ACIDIC FLUIDS ACROSS MARS: DETECTIONS OF MAGNESIUM-NICKEL SULFATES. A. S. Yen1, D. W. Ming2, R. Gellert3, D. W. Mittlefehldt2, E. B. Rampe4, D. T. Vaniman5, L. M. Thompson6, R. V. Morris2, B. C. Clark7, S. J. VanBommel3, R. E. Arvidson8, 1JPL- Caltech ([email protected]), 2NASA-JSC, 3University of Guelph, 4Aerodyne Industries, 5Planetary Science Institute, 6University of New Brunswick, 7Space Science Insti- tute, 7Washington University in St. Louis. Introduction: Calcium, magnesium and ferric iron sulfates have been detected by the instrument suites on the Mars rovers. A subset of the magnesium sulfates show clear associations with nickel. These associations indicate Ni2+ co-precipitation with or substitution for Mg2+ from sulfate-saturated solutions. Nickel is ex- tracted from primary rocks almost exclusively at pH values less than 6, constraining the formation of these Mg-Ni sulfates to mildly to strongly acidic conditions. There is clear evidence for aqueous alteration at the rim of Endeavour Crater (Meridiani Planum), in the Murray formation mudstone (Gale Crater), and near Home Plate (Gusev Crater). The discovery of Mg-Ni sulfates at these locations indicates a history of fluid- rock interactions at low pH. Fig 1: Histogram showing significant concentrations Mars Rovers: The Mars Exploration Rovers of sulfur in APXS analyses by the three Mars rovers (MER), Spirit and Opportunity, landed in January 2004 (mean value: 6.6%). at Gusev Crater and Meridiani Planum, respectively. Spirit traversed over 7.7 km through 2210 sols of sur- face operations, and Opportunity is currently on the degraded rim of Endeavour Crater after 4600 sols and 44 km of traverse. -

Raum-Zeit-Strategien Der Silbermöwe Larus Argentatus Und Verwandter Taxa Im Westlichen Ostseeraum - Dissertation Universität Rostock Von Ronald Klein 2001
Raum-Zeit-Strategien der Silbermöwe Larus argentatus und verwandter Taxa im westlichen Ostseeraum - Dissertation Universität Rostock von Ronald Klein 2001 Zusammenfassung In den Jahren 1991-1999 wurden in Mecklenburg-Vorpommern durch Ringablesung insgesamt 12448 Nachweise von 4808 verschiedenen Silbermöwen Larus argentatus erzielt, in anderen Bundesländern bzw. im Ausland erbrachten die Beringungen an nichtflüggen Individuen weitere 2052 Rückmeldungen. Durch den Einsatz von farbigen Kennringen mit individueller Inschrift ließ sich die Wiederfundrate bis auf 80 % steigern. Anhand dieses Ringfundmaterials wird eine detaillierte Quantifizierung nach Alter, geographischer Herkunft und Geschlecht in den einzelnen Zugperioden (Quartalen) vorgenommen. Die in Mecklenburg-Vorpommern erbrüteten Silbermöwen bleiben zu ca. 75% auch im Winter in der westlichen Ostsee, ca. 20 % der Individuen suchen das norddeutsche Binnenland im Bereich der großen Ballungszentren auf und nur etwa 5 % gelangen in ihrem ersten Winter an die Nordsee bis hin zum Pas-de-Calais. Das Binnenland wird in den Sommermonaten komplett geräumt, der größte Teil der Vögel wandert zurück, ein Teil sucht aber von dort aus die Nordseeküste auf und etabliert sich dort unter Umständen. Jährliche Wechsel in den Zugstrategien der Individuen sind dabei nicht ungewöhnlich. Der Anteil wegziehender Jungvögel unterscheidet sich nicht wesentlich von dem der Adulten, allerdings ist die auswärtige Aufenthaltsdauer bei Altvögeln wesentlich kürzer. Aus dem Großraum Rostock stammende Silbermöwen zeigen die geringste Tendenz zur Abwanderung, was aus den ganzjährig günstigen Existenzbedingungen in diesem Gebiet erklärt wird. Bei den Exemplaren von Rügen und der Wismar-Bucht ist die Zugneigung größer, wobei die erste Gruppe vergleichsweise in stärkerem Maße zur Nordsee tendiert. Erstmals wurde bei der Beringung der Nichtflüggen eine Zuordnung nach Geschlechtern durchgeführt. -

Vogelinsel Langenwerder – 100 Jahre Naturschutz –
U. Brenning und H. W. Nehls: Vogelinsel Langenwerder – 100 Jahre Naturschutz 1 Vogelinsel Langenwerder – 100 Jahre Naturschutz – Ringfundmitteilung der Vogelwarte Hiddensee Nr. 7/2012 Ulrich Brenning und Hans Wolfgang Nehls Herausgegeben im Auftrag des Vereins Langenwerder zum Schutz der Wat- und Wasservögel e. V. und der Ornithologischen Arbeitsgemeinschaft Mecklenburg-Vorpommern e.V. 2 Ornithol. Rundbr. Mecklenbg.-Vorpomm. 47, Sonderheft 2, 2013 U. Brenning und H. W. Nehls: Vogelinsel Langenwerder – 100 Jahre Naturschutz 3 GELEITWORT 100 Jahre Vogelschutz auf der Insel Langenwerder – eine Naturschutz war und ist niemals ein Selbstläufer und so ist lange Tradition und eine Erfolgsgeschichte des Natur- auch der Vogelschutz auf der Insel Langenwerder stets mit schutzes. dem Engagement meist ehrenamtlich tätiger Menschen, aber auch jahrzehntelang der Universität Rostock und oft Alles begann mit dem Schutz als Vogelfreistätte. Später, auch in Zusammenarbeit mit sachverständigen und ver- nach dem Inkrafttreten des ersten deutschen Naturschutz- ständnisvollen Behörden eng verbunden. Fast von Anfang gesetzes, wurde die Insel als eines der ersten Gebiete zum an musste der Schutz durch die stetige Anwesenheit von Naturschutzgebiet erklärt. Am Schutzstatus hat sich bis Vogelwärtern durchgesetzt werden. Auch daran hat sich heute nichts geändert, allerdings ist die kleine Insel seit bis heute nichts geändert. 20 Jahren integraler Bestandteil des weit umfassenderen Europäischen Vogelschutzgebietes „Wismarbucht und Möge das vorliegende Buch denjenigen, die die Insel Salzhaff“. Ihr an der mecklenburgischen Ostseeküste nach kennen, Anregung sein für neue Gedanken und Ideen zu wie vor einmaliger Brutbestand verschiedener Küstenvo- Schutz und Forschung, aber auch Ansporn sein im Bemü- gelarten zeichnet die Insel als eine Kernregion dieses euro- hen um den praktischen Erhalt der Insel. -

Landkreis Vorpommern-Rügen Der Landrat
Landkreis Vorpommern-Rügen Der Landrat Hinweis: Zusätzlich zu dem unten bezeichneten Risikogebiet besteht in den derzeit gülti• gen Restriktionszonen (Sperrbezirk, Beobachtungsgebiet) ebenfalls die Stallpflicht für Geflügel. Informieren Sie sich zu den betroffenen Ortsteilen und Gemeinden unter www.lk-vr.dein der Zusammenfassung der Restriktions- und Aufstallungsgebiete. Der Landrat des LandkreisesVorpommern-Rügenerlässt folgende Allgemei nverfügung zur Änderung der Tierseuchenverfügung zur Aufstallung von Geflügel zum Schutz gegen die Klassische Geflügelpest vom 11.11.2016 1. Ziffer 1 der Allgemeinverfügung - Tierseuchenverfügung zur Aufstallung von Geflügel zum Schutz gegendie KlassischeGeflügelpest vom 11.11.2016 wird durch folgende Ziffer 1 er• setzt: 1. In den folgenden Gebieten: die Gemeinden und Städte insgesamt: Altenpleen, Barth, Fuhlendorf, Groß Kordshagen, Groß Mohrdorf, Kenz-Küstrow, Klausdorf, Kramerhof, Neu Bartelshagen, Preetz, Prohn, Pruchten, Putbus, Saal, Sassnitz, Stralsund einschließlich Dänholm, Wendorf von der Gemeinde Zingst: das bebaute Gemeindegebiet der Ortschaft Zingst einschließ• lich Müggenburg und Insel Kirr, begrenzt im Westen durch das Waldgebiet des Freesen• bruchs, im Osten durch den Osterwald sowie die Küstenlinien im Norden und Süden in der Gemeinde Wieck a. d. Darß: innerhalb der Gemeindegrenzen, im Osten jedoch nur bis zur Höhedes Klärwerks und des Jagdhauses,begrenzt durch den Lauf des Kanals von der Gemeinde Born a. d. Darß: das bebaute Gemeindegebiet der Ortschaft Born a. Darß einschließlich der