Downloaded 10/2/2021 2:34:47 AM
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Contrasting Patterns of Andean Diversification Among Three Diverse Clades of Neotropical Clearwing Butterflies
Contrasting patterns of Andean diversification among three diverse clades of Neotropical clearwing butterflies The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Chazot, Nicolas, Donna Lisa De#Silva, Keith R. Willmott, André V. L. Freitas, Gerardo Lamas, James Mallet, Carlos E. Giraldo, Sandra Uribe, and Marianne Elias. 2018. “Contrasting patterns of Andean diversification among three diverse clades of Neotropical clearwing butterflies.” Ecology and Evolution 8 (8): 3965-3982. doi:10.1002/ ece3.3622. http://dx.doi.org/10.1002/ece3.3622. Published Version doi:10.1002/ece3.3622 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:37160427 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA Received: 12 April 2017 | Revised: 31 August 2017 | Accepted: 11 October 2017 DOI: 10.1002/ece3.3622 ORIGINAL RESEARCH Contrasting patterns of Andean diversification among three diverse clades of Neotropical clearwing butterflies Nicolas Chazot1,2,* | Donna Lisa De-Silva2,* | Keith R. Willmott3 | André V. L. Freitas4 | Gerardo Lamas5 | James Mallet6 | Carlos E. Giraldo7 | Sandra Uribe8 | Marianne Elias2 1Department of Biology, Lunds Universitet, Lund, Sweden 2Institut de Systématique, Évolution, Biodiversité, ISYEB-UMR 7205–CNRS MNHN UPMC EPHE, Muséum national -

Lepidopterofauna (Papilionoidea E Hesperioidea) Do Parque Estadual Do Chandless E Arredores, Acre, Brasil
Biota Neotrop., vol. 10, no. 4 Lepidopterofauna (Papilionoidea e Hesperioidea) do Parque Estadual do Chandless e arredores, Acre, Brasil Olaf Hermann Hendrik Mielke1,2, Eduardo Carneiro¹ & Mirna Martins Casagrande¹ ¹Laboratório de Estudos de Lepidoptera Neotropical, Departamento de Zoologia, Universidade Federal do Paraná – UFPR, CP 19020, CEP 81531-980, Curitiba, PR, Brasil 2Autor para correspondência: Olaf Hermann Hendrik Mielke, e-mail: [email protected] MIELKE, O.H.H., CARNEIRO, E. & CASAGRANDE, M.M. Lepidopterofauna (Papilionoidea e Hesperioidea) of the Parque Estadual do Chandless and surroundings, Acre, Brazil. Biota Neotrop. 10(4): http://www. biotaneotropica.org.br/v10n4/en/abstract?inventory+bn03210042010. Abstract: Given the absence of Lepidoptera inventories in the State of Acre and its scarcity in the Brazilian Amazon forest, this study aimed to list the species of Hesperioidea and Papilionoidea present in the Parque Estadual do Chandless and surroundings. The access to the region is complicated and it has no infrastructure for scientific research. During 14 days, the butterflies were collected with entomological nets, traps and Ahrenholz’s technique in different environments in the park and its surroundings. A total of 482 species were identified, none of them present in red lists of endangered species. It is expected a significantly greater number of species after the addition of new collections in other seasons, as the Jacknife 1 estimate does not reach its asymptote, or as compared to inventories in nearby areas that list nearly 1700 species after a greater sampling effort. Keywords: amazonian forest, butterflies, inventory, protected area. MIELKE, O.H.H., CARNEIRO, E. & CASAGRANDE, M.M. Lepidopterofauna (Papilionoidea e Hesperioidea) do Parque Estadual do Chandless e arredores, Acre, Brasil. -

INSECTA: LEPIDOPTERA) DE GUATEMALA CON UNA RESEÑA HISTÓRICA Towards a Synthesis of the Papilionoidea (Insecta: Lepidoptera) from Guatemala with a Historical Sketch
ZOOLOGÍA-TAXONOMÍA www.unal.edu.co/icn/publicaciones/caldasia.htm Caldasia 31(2):407-440. 2009 HACIA UNA SÍNTESIS DE LOS PAPILIONOIDEA (INSECTA: LEPIDOPTERA) DE GUATEMALA CON UNA RESEÑA HISTÓRICA Towards a synthesis of the Papilionoidea (Insecta: Lepidoptera) from Guatemala with a historical sketch JOSÉ LUIS SALINAS-GUTIÉRREZ El Colegio de la Frontera Sur (ECOSUR). Unidad Chetumal. Av. Centenario km. 5.5, A. P. 424, C. P. 77900. Chetumal, Quintana Roo, México, México. [email protected] CLAUDIO MÉNDEZ Escuela de Biología, Universidad de San Carlos, Ciudad Universitaria, Campus Central USAC, Zona 12. Guatemala, Guatemala. [email protected] MERCEDES BARRIOS Centro de Estudios Conservacionistas (CECON), Universidad de San Carlos, Avenida La Reforma 0-53, Zona 10, Guatemala, Guatemala. [email protected] CARMEN POZO El Colegio de la Frontera Sur (ECOSUR). Unidad Chetumal. Av. Centenario km. 5.5, A. P. 424, C. P. 77900. Chetumal, Quintana Roo, México, México. [email protected] JORGE LLORENTE-BOUSQUETS Museo de Zoología, Facultad de Ciencias, UNAM. Apartado Postal 70-399, México D.F. 04510; México. [email protected]. Autor responsable. RESUMEN La riqueza biológica de Mesoamérica es enorme. Dentro de esta gran área geográfi ca se encuentran algunos de los ecosistemas más diversos del planeta (selvas tropicales), así como varios de los principales centros de endemismo en el mundo (bosques nublados). Países como Guatemala, en esta gran área biogeográfi ca, tiene grandes zonas de bosque húmedo tropical y bosque mesófi lo, por esta razón es muy importante para analizar la diversidad en la región. Lamentablemente, la fauna de mariposas de Guatemala es poco conocida y por lo tanto, es necesario llevar a cabo un estudio y análisis de la composición y la diversidad de las mariposas (Lepidoptera: Papilionoidea) en Guatemala. -

Butterflies (Lepidoptera: Papilionoidea) in a Coastal Plain Area in the State of Paraná, Brazil
62 TROP. LEPID. RES., 26(2): 62-67, 2016 LEVISKI ET AL.: Butterflies in Paraná Butterflies (Lepidoptera: Papilionoidea) in a coastal plain area in the state of Paraná, Brazil Gabriela Lourenço Leviski¹*, Luziany Queiroz-Santos¹, Ricardo Russo Siewert¹, Lucy Mila Garcia Salik¹, Mirna Martins Casagrande¹ and Olaf Hermann Hendrik Mielke¹ ¹ Laboratório de Estudos de Lepidoptera Neotropical, Departamento de Zoologia, Universidade Federal do Paraná, Caixa Postal 19.020, 81.531-980, Curitiba, Paraná, Brazil Corresponding author: E-mail: [email protected]٭ Abstract: The coastal plain environments of southern Brazil are neglected and poorly represented in Conservation Units. In view of the importance of sampling these areas, the present study conducted the first butterfly inventory of a coastal area in the state of Paraná. Samples were taken in the Floresta Estadual do Palmito, from February 2014 through January 2015, using insect nets and traps for fruit-feeding butterfly species. A total of 200 species were recorded, in the families Hesperiidae (77), Nymphalidae (73), Riodinidae (20), Lycaenidae (19), Pieridae (7) and Papilionidae (4). Particularly notable records included the rare and vulnerable Pseudotinea hemis (Schaus, 1927), representing the lowest elevation record for this species, and Temenis huebneri korallion Fruhstorfer, 1912, a new record for Paraná. These results reinforce the need to direct sampling efforts to poorly inventoried areas, to increase knowledge of the distribution and occurrence patterns of butterflies in Brazil. Key words: Atlantic Forest, Biodiversity, conservation, inventory, species richness. INTRODUCTION the importance of inventories to knowledge of the fauna and its conservation, the present study inventoried the species of Faunal inventories are important for providing knowledge butterflies of the Floresta Estadual do Palmito. -

Tropical Forests of Brazil and Their Lepidoptera
1959 Journal of the Lepido plerists' Society 79 ESPECIALLY FOR FIELD COLLECTORS (Under the supervision of FRED T. THORNE, 1360 Merritt Dr., El Cajon, Calif., U.S.A.) FIRST IMPRESSIONS OF THE TROPICAL FORESTS OF SOUTHEASTERN BRAZIL AND THEIR LEPIDOPTERA by E. P. WILTSHIRE Before leaving England for Rio de Janeiro, I had noted that several subscribers of the Lepidopterists' Society inhabited that city; some of these were private citizens, others employees of at least two scientific institutions. A rapid glance at Seitz, Macrolepidoptera of the fVorld, Vol. 5, had shewed me that the neighbourhood of Rio was a favourite collecting ground for Lepi doptera. It looked as though I should not be able to make any valuable scien tific discoveries during a stay of a few years there, but that my outlook would be broadened. Now, after a year at Rio, during which all too little time could be spared for entomology, I venture to summarise my impressions of the Lepi doptera of the city and its neighbourhood and of general conditions affecting their life and their study, in the hope that these may interest readers outside Brazil. They fall into the following subject headings: The study: state of knowledge. The butterfly industry. The habitat: state of botanical knowledge. Representation of groups of Lepidoptera. Characteristic patterns, including mimetic and melanistic; extreme adap- tations. Phenology. Character of the fauna. Breeding, catching, and keeping. THE STUDY OF LEPIDOPTERA AND THE STATE OF KNOWLEDGE Rio is a city of about three million inhabitants. I t is the federal capital of one of the largest countries in the world. -
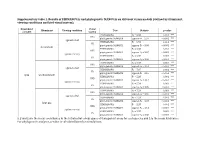
Supplementary Table 1. Results of Permanovas and Phylogenetic Manovas on Different Vision Models (Defined by Illuminant, Viewing Conditions and Bird Visual System)
Supplementary table 1. Results of PERMANOVAs and phylogenetic MANOVAs on different vision models (defined by illuminant, viewing conditions and bird visual system). Dependent Visual Illuminant Viewing condition Test Statistic p-value variable system PERMANOVA F9 = 6.88 0.001 *** UVS phylogenetic MANOVA approx-F9 = 2.97 < 0.001 *** against a leaf PERMANOVA F9 = 6.93 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.05 < 0.001 *** forest shade PERMANOVA F9 = 5.38 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.07 < 0.001 *** against the sky PERMANOVA F9 = 5.38 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.36 < 0.001 *** PERMANOVA F9 = 7.04 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.01 < 0.001 *** against a leaf PERMANOVA F9 = 7.07 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.10 < 0.001 *** xyzL woodland shade PERMANOVA F9 = 5.33 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.12 < 0.001 *** against the sky PERMANOVA F9 = 5.34 0.002 ** VS phylogenetic MANOVA approx-F9 = 3.39 < 0.001 *** PERMANOVA F9 = 7.24 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.00 < 0.001 *** against a leaf PERMANOVA F9 = 7.24 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.07 < 0.001 *** large gap PERMANOVA F9 = 5.37 0.001 *** UVS phylogenetic MANOVA approx-F9 = 3.14 < 0.001 *** against the sky PERMANOVA F9 = 5.37 0.001 *** VS phylogenetic MANOVA approx-F9 = 3.38 < 0.001 *** x, y and z are the mean coordinates in the tetrahedral colour space of transparent areas for each species and L is the mean luminance. -

Re-Emergence and Diversification of a Specialised Antennal Lobe Morphology in Ithomiine Butterflies
bioRxiv preprint doi: https://doi.org/10.1101/2020.10.13.336206; this version posted October 13, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Re-emergence and diversification of a specialised antennal lobe morphology 2 in ithomiine butterflies 3 4 Authors: 5 Billy J Morris1*, Antoine Couto1,2, Asli Aydin3, Stephen H Montgomery2*. 6 7 Affiliations: 1 8 Dept. of Zoology, University of Cambridge, Downing Street, Cambridge, CB2 3EJ 9 2 School of Biological Sciences, University of Bristol, 24 Tyndall Avenue, Bristol, BS8 1TQ 10 3 School of Medicine, Koc University, Rumelifeneri Yolu 34450 Sarıyer / Istanbul, Turkey 11 12 * corresponding authors: 13 BJM: [email protected] 14 SHM: [email protected] 15 16 Abstract 17 How an organism’s sensory system functions is central to how it navigates its environment and 18 meets the behavioural challenges associated with survival and reproduction. Comparing sensory 19 systems across species can reveal how facets of behaviour and ecology promote adaptive shifts 20 in the relative importance of certain environmental cues. The insect olfactory system is prominent 21 model for investigating how ecological factors impact sensory reception and processing. Notably 22 work in Lepidoptera led to the discovery of vastly expanded structures, termed a macroglomerular 23 complex (MGC), within the primary olfactory processing centre. These structures typically process 24 pheromonal cues and provide a classic example of how variation in size can influence the 25 functional processing of sensory cues. -

Rev Iss Web Mec 13773 25-22 5765..5784
Molecular Ecology (2016) 25, 5765–5784 doi: 10.1111/mec.13773 Into the Andes: multiple independent colonizations drive montane diversity in the Neotropical clearwing butterflies Godyridina NICOLAS CHAZOT,*† KEITH R. WILLMOTT,‡ FABIEN L. CONDAMINE,§¶ DONNA LISA DE-SILVA,* ANDREV.L.FREITAS,**GERARDOLAMAS,†† HELENE MORLON,‡‡ CARLOS E. GIRALDO,§§ CHRIS D. JIGGINS,¶¶ MATHIEU JORON,*** JAMES MALLET,††† SANDRA URIBE‡‡‡ and MARIANNE ELIAS* *Institut de Systematique, Evolution, Biodiversite, ISYEB – UMR 7205 – CNRS MNHN UPMC EPHE, Museum national d’Histoire naturelle, Sorbonne Universites, 57 rue Cuvier CP50, F-75005 Paris, France, †Department of Biology, University of Lund, 223 62 Lund, Sweden, ‡McGuire Center for Lepidoptera and Biodiversity, Florida Museum of Natural History, University of Florida, Gainesville, FL 32611, USA, §CNRS, UMR 5554 Institut des Sciences de l’Evolution (Universite de Montpellier), Place Eugene Bataillon, 34095 Montpellier, France, ¶Department of Biological Sciences, University of Alberta, T6G 2E9 Edmonton, AB, Canada, **Departamento de Zoologia and Museu de Zoologia, Instituto de Biologia, Universidade Estadual de Campinas, Campinas, S~ao Paulo, Brazil, ††Museo de Historia Natural, Universidad Nacional de San Marcos, Lima, Peru, ‡‡IBENS, Ecole Normale Superieure, UMR 8197 CNRS, Paris, France, §§Grupo de Investigacion de Sanidad Vegetal, Universidad Catolica de Oriente, Rionegro, Antioquia, Colombia, ¶¶Department of Zoology, University of Cambridge, Cambridge, UK, ***Centre d’Ecologie Fonctionnelle et Evolutive, CEFE, UMR 5175 CNRS – EPHE – Universite de Montpellier – Universite Paul Valery Montpellier, 34293 Montpellier 5, France, †††Department of Organismic and Evolutionary Biology, Harvard University, Cambridge, MA 02138, USA, ‡‡‡Universidad Nacional de Colombia, sede Medellın, Medellın, Colombia Abstract Understanding why species richness peaks along the Andes is a fundamental question in the study of Neotropical biodiversity. -

Effects of Land Use on Butterfly (Lepidoptera: Nymphalidae) Abundance and Diversity in the Tropical Coastal Regions of Guyana and Australia
ResearchOnline@JCU This file is part of the following work: Sambhu, Hemchandranauth (2018) Effects of land use on butterfly (Lepidoptera: Nymphalidae) abundance and diversity in the tropical coastal regions of Guyana and Australia. PhD Thesis, James Cook University. Access to this file is available from: https://doi.org/10.25903/5bd8e93df512e Copyright © 2018 Hemchandranauth Sambhu The author has certified to JCU that they have made a reasonable effort to gain permission and acknowledge the owners of any third party copyright material included in this document. If you believe that this is not the case, please email [email protected] EFFECTS OF LAND USE ON BUTTERFLY (LEPIDOPTERA: NYMPHALIDAE) ABUNDANCE AND DIVERSITY IN THE TROPICAL COASTAL REGIONS OF GUYANA AND AUSTRALIA _____________________________________________ By: Hemchandranauth Sambhu B.Sc. (Biology), University of Guyana, Guyana M.Sc. (Res: Plant and Environmental Sciences), University of Warwick, United Kingdom A thesis Prepared for the College of Science and Engineering, in partial fulfillment of the requirements for the degree of Doctor of Philosophy James Cook University February, 2018 DEDICATION ________________________________________________________ I dedicate this thesis to my wife, Alliea, and to our little girl who is yet to make her first appearance in this world. i ACKNOWLEDGEMENTS ________________________________________________________ I would like to thank the Australian Government through their Department of Foreign Affairs and Trade for graciously offering me a scholarship (Australia Aid Award – AusAid) to study in Australia. From the time of my departure from my home country in 2014, Alex Salvador, Katherine Elliott and other members of the AusAid team have always ensured that the highest quality of care was extended to me as a foreign student in a distant land. -

(Coleoptera) of Australia
AUSTRALIAN MUSEUM SCIENTIFIC PUBLICATIONS McKeown, K. C., 1947. Catalogue of the Cerambycidae (Coleoptera) of Australia. Australian Museum Memoir 10: 1–190. [2 May 1947]. doi:10.3853/j.0067-1967.10.1947.477 ISSN 0067-1967 Published by the Australian Museum, Sydney naturenature cultureculture discover discover AustralianAustralian Museum Museum science science is is freely freely accessible accessible online online at at www.australianmuseum.net.au/publications/www.australianmuseum.net.au/publications/ 66 CollegeCollege Street,Street, SydneySydney NSWNSW 2010,2010, AustraliaAustralia THE AUSTRALIAN MUSEUM, SYDNEY MEMOIR X. CATALOGUE OF THE CERAMBYCIDAE (COLEOPTERA) OF AUSTRALIA BY KEITH C. McKEOWN, F.R.Z.S., Assistant Entomologist. The Australian Museum. PUBLISHED BY ORDER OF THE TRUSTEES A. B. Walkom, D.%., Director. Sydney, May 2, I947 PREFACE. The accompanying Catalogue of the Cerambycidae is the first, dealing solely with Australian genera and species, to be published since that of Pascoe in 1867. Masters' Catalogue of the Described Coleoptera of Australia, 1885-1887, included the Cerambycidae, and was based on the work of Gemminger and Harold. A new catalogue has been badly needed owing to the large number of new species described in recent years, and the changes in the already complicated synonymy. The Junk catalogue, covering the Coleoptera of the world, is defective in many respects, as well as being too unwieldy, and too costly for the average Australian worker. Many of the references in the Junk catalogue are inaccurate, synonymy misleading, and the genera under which the species were originally described omitted, and type localities are not quoted. In this catalogue every care has been taken to ensure accuracy, and the fact that it has been used, in slip form, over a number. -

Nymphalidae: Ithomiinae)
STUDIES ON THE ECOLOGY AND EVOLUTION OF NEOTROPICAL ITHOMIINE BUTTERFLIES (NYMPHALIDAE: ITHOMIINAE) by GEORGE WILLIAM BECCALONI A thesis submitted for the degree of Doctor ofPhilosophy ofthe University ofLondon October 1995 Biogeography and Conservation Laboratory Centre for Population Biology Department of Entomology Imperial College The Natural History Museum Silwood Park Cromwell Road Ascot London SW7 5BD Berkshire SL5 7PY 2 To my mother, Benjie & Judy in love and gratitude 3 ABSTRACT Two aspects ofthe ecology ofNeotropical ithomiine butterflies (Nymphalidae: Ithomiinae) are discussed: mimicry (Chapters 2, 3) and species richness (Chapters 4, 5). Chapter 2 defines eight mimicry complexes involving ithomiines and other insects found in eastern Ecuador. These complexes are dominated by ithomiine individuals. Hypotheses to explain polymorphism in Batesian and Mullerian mimics are assessed. In Chapter 3, evidence that sympatric ithomiine-dominated mimicry complexes are segregated by microhabitat is reviewed. Data confirm that sympatric complexes are segregated vertically by flight height. Flight height is shown to be positively correlated with larval host-plant height. Host-plant partitioning between species in a butterfly community results in the formation of microhabitat guilds of species, and evidence suggests that mimicry may evolve between species which share a guild, but not between guilds. Models for the evolution of mimicry complexes in sympatry, and for polymorphism and dual sex-limited mimicry in Mullerian mimics, are discussed in the light of these findings. Chapter 4 investigates relationships between species richness offamilies and subfamilies ofNeotropical butterflies and overall butterfly species richness at local and regional scales. A strong positive correlation is demonstrated between ithomiine richness and the species richness of all other butterflies. -

Issue Full File
BİLGE INTERNATIONAL JOURNAL OF SCIENCE AND TECHNOLOGY RESEARCH VOLUME: 4 ISSUE: 2 2020 ISSN: 2651-401X e-ISSN: 2651-4028 Owner: Dr. Hamza KANDEMİR Editor in Chief: Prof. Dr. Kürşad ÖZKAN Co-Editor: Editorial Advisory Board: Editorial Board: Dr. Mustafa KARABOYACI Ahmet AKSOY, Prof. Dr. Ali Cesur ONMAZ, Assoc. Prof. Dr. Akdeniz University, Turkey Erciyes University, Turkey Technical Editors: Res. Asst. Abdullah BERAM Amer KANAN, Prof. Dr. Asko Tapio LEHTİJÄRVİ, Assoc. Prof. Dr. Instructor Serkan ÖZDEMİR Al-Quds University, Palestine Bursa Technical University, Turkey Cüneyt ÇIRAK, Prof. Dr. Halil GÖKÇE, Assoc. Prof. Dr. Layout Editors: Ondokuz Mayıs University, Turkey Giresun University, Turkey Instructor Doğan AKDEMİR MSc. Tunahan ÇINAR Ender MAKİNECİ, Prof. Dr. Kubilay AKÇAÖZOĞLU, Assoc. Prof. Dr. İstanbul University, Turkey Niğde Ömer Halisdemir University, Turkey Cover designer: Instructor Serkan ÖZDEMİR Gülcan ÖZKAN, Prof. Dr. Şule Sultan UĞUR, Assoc. Prof. Dr. Süleyman Demirel University, Turkey Suleyman Demirel University, Turkey Press: Kutbilge Association of Academicians İbrahim ÖZDEMİR, Prof. Dr. Ahmet MERT, Assoc. Prof. Dr. Distribution, Sales, Publisher; Certificate Isparta University of Applied Sciences, Turkey No: 42086 Isparta University of Applied Sciences, Turkey 32040, Isparta, TURKEY Kari HELİÖVAARA, Prof. Dr. Ayşe KOCABIYIK, Asst. Prof. Dr. University of Helsinki, Finland Suleyman Demirel University, Turkey Contact: Kutbilge Association of Academicians, Kırali MÜRTEZAOĞLU, Prof. Dr. Fecir DURAN, Asst. Prof. Dr. 32040, Isparta, TURKEY Gazi University, Turkey Gazi University, Turkey Web : dergipark.gov.tr/bilgesci Mehmet KILIÇ, Prof. Dr. Kubilay TAŞDELEN, Asst. Prof. Dr. E-mail : [email protected] Suleyman Demirel University, Turkey Suleyman Demirel University, Turkey Mehmet KİTİŞ, Prof. Dr. Nuri ÖZTÜRK, Asst. Prof. Dr. Suleyman Demirel University, Turkey Giresun University, Turkey Mohamed Lahbib BEN JAMAA, Prof.