Covered for One PFT Two Loci for Both FEV1 and FVC (Table 2, Fig
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

An Integrative Systems Biology Approach Identifies Molecular
Journal of Clinical Medicine Article An Integrative Systems Biology Approach Identifies Molecular Signatures Associated with Gallbladder Cancer Pathogenesis Nabanita Roy 1 , Mrinmoy Kshattry 1, Susmita Mandal 2, Mohit Kumar Jolly 2 , Dhruba Kumar Bhattacharyya 3 and Pankaj Barah 1,* 1 Department of Molecular Biology and Biotechnology, Tezpur University, Sonitpur 784028, India; [email protected] (N.R.); [email protected] (M.K.) 2 Centre for BioSystems Science and Engineering, Indian Institute of Science, Bangalore 560012, India; [email protected] (S.M.); [email protected] (M.K.J.) 3 Department of Computer Science and Engineering, Tezpur University, Sonitpur 784028, India; [email protected] * Correspondence: [email protected]; Tel.: +91-3712-27-5415 Abstract: Gallbladder cancer (GBC) has a lower incidence rate among the population relative to other cancer types but is a major contributor to the total number of biliary tract system cancer cases. GBC is distinguished from other malignancies by its high mortality, marked geographical variation and poor prognosis. To date no systemic targeted therapy is available for GBC. The main objective of this study is to determine the molecular signatures correlated with GBC development using integrative systems level approaches. We performed analysis of publicly available transcriptomic data to identify differentially regulated genes and pathways. Differential co-expression network analysis and transcriptional regulatory network analysis was performed to identify hub genes and Citation: Roy, N.; Kshattry, M.; hub transcription factors (TFs) associated with GBC pathogenesis and progression. Subsequently, we Mandal, S.; Jolly, M.K.; Bhattacharyya, assessed the epithelial-mesenchymal transition (EMT) status of the hub genes using a combination D.K.; Barah, P. -

Whole Exome Sequencing in Families at High Risk for Hodgkin Lymphoma: Identification of a Predisposing Mutation in the KDR Gene
Hodgkin Lymphoma SUPPLEMENTARY APPENDIX Whole exome sequencing in families at high risk for Hodgkin lymphoma: identification of a predisposing mutation in the KDR gene Melissa Rotunno, 1 Mary L. McMaster, 1 Joseph Boland, 2 Sara Bass, 2 Xijun Zhang, 2 Laurie Burdett, 2 Belynda Hicks, 2 Sarangan Ravichandran, 3 Brian T. Luke, 3 Meredith Yeager, 2 Laura Fontaine, 4 Paula L. Hyland, 1 Alisa M. Goldstein, 1 NCI DCEG Cancer Sequencing Working Group, NCI DCEG Cancer Genomics Research Laboratory, Stephen J. Chanock, 5 Neil E. Caporaso, 1 Margaret A. Tucker, 6 and Lynn R. Goldin 1 1Genetic Epidemiology Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, NIH, Bethesda, MD; 2Cancer Genomics Research Laboratory, Division of Cancer Epidemiology and Genetics, National Cancer Institute, NIH, Bethesda, MD; 3Ad - vanced Biomedical Computing Center, Leidos Biomedical Research Inc.; Frederick National Laboratory for Cancer Research, Frederick, MD; 4Westat, Inc., Rockville MD; 5Division of Cancer Epidemiology and Genetics, National Cancer Institute, NIH, Bethesda, MD; and 6Human Genetics Program, Division of Cancer Epidemiology and Genetics, National Cancer Institute, NIH, Bethesda, MD, USA ©2016 Ferrata Storti Foundation. This is an open-access paper. doi:10.3324/haematol.2015.135475 Received: August 19, 2015. Accepted: January 7, 2016. Pre-published: June 13, 2016. Correspondence: [email protected] Supplemental Author Information: NCI DCEG Cancer Sequencing Working Group: Mark H. Greene, Allan Hildesheim, Nan Hu, Maria Theresa Landi, Jennifer Loud, Phuong Mai, Lisa Mirabello, Lindsay Morton, Dilys Parry, Anand Pathak, Douglas R. Stewart, Philip R. Taylor, Geoffrey S. Tobias, Xiaohong R. Yang, Guoqin Yu NCI DCEG Cancer Genomics Research Laboratory: Salma Chowdhury, Michael Cullen, Casey Dagnall, Herbert Higson, Amy A. -

Nuclear PTEN Safeguards Pre-Mrna Splicing to Link Golgi Apparatus for Its Tumor Suppressive Role
ARTICLE DOI: 10.1038/s41467-018-04760-1 OPEN Nuclear PTEN safeguards pre-mRNA splicing to link Golgi apparatus for its tumor suppressive role Shao-Ming Shen1, Yan Ji2, Cheng Zhang1, Shuang-Shu Dong2, Shuo Yang1, Zhong Xiong1, Meng-Kai Ge1, Yun Yu1, Li Xia1, Meng Guo1, Jin-Ke Cheng3, Jun-Ling Liu1,3, Jian-Xiu Yu1,3 & Guo-Qiang Chen1 Dysregulation of pre-mRNA alternative splicing (AS) is closely associated with cancers. However, the relationships between the AS and classic oncogenes/tumor suppressors are 1234567890():,; largely unknown. Here we show that the deletion of tumor suppressor PTEN alters pre-mRNA splicing in a phosphatase-independent manner, and identify 262 PTEN-regulated AS events in 293T cells by RNA sequencing, which are associated with significant worse outcome of cancer patients. Based on these findings, we report that nuclear PTEN interacts with the splicing machinery, spliceosome, to regulate its assembly and pre-mRNA splicing. We also identify a new exon 2b in GOLGA2 transcript and the exon exclusion contributes to PTEN knockdown-induced tumorigenesis by promoting dramatic Golgi extension and secretion, and PTEN depletion significantly sensitizes cancer cells to secretion inhibitors brefeldin A and golgicide A. Our results suggest that Golgi secretion inhibitors alone or in combination with PI3K/Akt kinase inhibitors may be therapeutically useful for PTEN-deficient cancers. 1 Department of Pathophysiology, Key Laboratory of Cell Differentiation and Apoptosis of Chinese Ministry of Education, Shanghai Jiao Tong University School of Medicine (SJTU-SM), Shanghai 200025, China. 2 Institute of Health Sciences, Shanghai Institutes for Biological Sciences of Chinese Academy of Sciences and SJTU-SM, Shanghai 200025, China. -

Appendix 2. Significantly Differentially Regulated Genes in Term Compared with Second Trimester Amniotic Fluid Supernatant
Appendix 2. Significantly Differentially Regulated Genes in Term Compared With Second Trimester Amniotic Fluid Supernatant Fold Change in term vs second trimester Amniotic Affymetrix Duplicate Fluid Probe ID probes Symbol Entrez Gene Name 1019.9 217059_at D MUC7 mucin 7, secreted 424.5 211735_x_at D SFTPC surfactant protein C 416.2 206835_at STATH statherin 363.4 214387_x_at D SFTPC surfactant protein C 295.5 205982_x_at D SFTPC surfactant protein C 288.7 1553454_at RPTN repetin solute carrier family 34 (sodium 251.3 204124_at SLC34A2 phosphate), member 2 238.9 206786_at HTN3 histatin 3 161.5 220191_at GKN1 gastrokine 1 152.7 223678_s_at D SFTPA2 surfactant protein A2 130.9 207430_s_at D MSMB microseminoprotein, beta- 99.0 214199_at SFTPD surfactant protein D major histocompatibility complex, class II, 96.5 210982_s_at D HLA-DRA DR alpha 96.5 221133_s_at D CLDN18 claudin 18 94.4 238222_at GKN2 gastrokine 2 93.7 1557961_s_at D LOC100127983 uncharacterized LOC100127983 93.1 229584_at LRRK2 leucine-rich repeat kinase 2 HOXD cluster antisense RNA 1 (non- 88.6 242042_s_at D HOXD-AS1 protein coding) 86.0 205569_at LAMP3 lysosomal-associated membrane protein 3 85.4 232698_at BPIFB2 BPI fold containing family B, member 2 84.4 205979_at SCGB2A1 secretoglobin, family 2A, member 1 84.3 230469_at RTKN2 rhotekin 2 82.2 204130_at HSD11B2 hydroxysteroid (11-beta) dehydrogenase 2 81.9 222242_s_at KLK5 kallikrein-related peptidase 5 77.0 237281_at AKAP14 A kinase (PRKA) anchor protein 14 76.7 1553602_at MUCL1 mucin-like 1 76.3 216359_at D MUC7 mucin 7, -

Unique Protein Expression Signatures of Survival Time in Kidney Renal Clear
The Author(s) BMC Genomics 2017, 18(Suppl 6):678 DOI 10.1186/s12864-017-4026-6 RESEARCH Open Access Unique protein expression signatures of survival time in kidney renal clear cell carcinoma through a pan-cancer screening Guangchun Han1, Wei Zhao2, Xiaofeng Song3, Patrick Kwok-Shing Ng4, Jose A. Karam5, Eric Jonasch6, Gordon B. Mills2, Zhongming Zhao1,7*, Zhiyong Ding2* and Peilin Jia1* From The International Conference on Intelligent Biology and Medicine (ICIBM) 2016 Houston, TX, USA. 08-10 December 2016 Abstract Background: In 2016, it is estimated that there will be 62,700 new cases of kidney cancer in the United States, and 14,240 patients will die from the disease. Because the incidence of kidney renal clear cell carcinoma (KIRC), the most common type of kidney cancer, is expected to continue to increase in the US, there is an urgent need to find effective diagnostic biomarkers for KIRC that could help earlier detection of and customized treatment strategies for the disease. Accordingly, in this study we systematically investigated KIRC’s prognostic biomarkers for survival using the reverse phase protein array (RPPA) data and the high throughput sequencing data from The Cancer Genome Atlas (TCGA). Results: With comprehensive data available in TCGA, we systematically screened protein expression based survival biomarkers in 10 major cancer types, among which KIRC presented many protein prognostic biomarkers of survival time. This is in agreement with a previous report that expression level changes (mRNAs, microRNA and protein) may have a better performance for prognosis of KIRC. In this study, we also identified 52 prognostic genes for KIRC, many of which are involved in cell-cycle and cancer signaling, as well as 15 tumor-stage-specific prognostic biomarkers. -

The Landscape of Human Mutually Exclusive Splicing
bioRxiv preprint doi: https://doi.org/10.1101/133215; this version posted May 2, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. The landscape of human mutually exclusive splicing Klas Hatje1,2,#,*, Ramon O. Vidal2,*, Raza-Ur Rahman2, Dominic Simm1,3, Björn Hammesfahr1,$, Orr Shomroni2, Stefan Bonn2§ & Martin Kollmar1§ 1 Group of Systems Biology of Motor Proteins, Department of NMR-based Structural Biology, Max-Planck-Institute for Biophysical Chemistry, Göttingen, Germany 2 Group of Computational Systems Biology, German Center for Neurodegenerative Diseases, Göttingen, Germany 3 Theoretical Computer Science and Algorithmic Methods, Institute of Computer Science, Georg-August-University Göttingen, Germany § Corresponding authors # Current address: Roche Pharmaceutical Research and Early Development, Pharmaceutical Sciences, Roche Innovation Center Basel, F. Hoffmann-La Roche Ltd., Basel, Switzerland $ Current address: Research and Development - Data Management (RD-DM), KWS SAAT SE, Einbeck, Germany * These authors contributed equally E-mail addresses: KH: [email protected], RV: [email protected], RR: [email protected], DS: [email protected], BH: [email protected], OS: [email protected], SB: [email protected], MK: [email protected] - 1 - bioRxiv preprint doi: https://doi.org/10.1101/133215; this version posted May 2, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. -

Chemical Agent and Antibodies B-Raf Inhibitor RAF265
Supplemental Materials and Methods: Chemical agent and antibodies B-Raf inhibitor RAF265 [5-(2-(5-(trifluromethyl)-1H-imidazol-2-yl)pyridin-4-yloxy)-N-(4-trifluoromethyl)phenyl-1-methyl-1H-benzp{D, }imidazol-2- amine] was kindly provided by Novartis Pharma AG and dissolved in solvent ethanol:propylene glycol:2.5% tween-80 (percentage 6:23:71) for oral delivery to mice by gavage. Antibodies to phospho-ERK1/2 Thr202/Tyr204(4370), phosphoMEK1/2(2338 and 9121)), phospho-cyclin D1(3300), cyclin D1 (2978), PLK1 (4513) BIM (2933), BAX (2772), BCL2 (2876) were from Cell Signaling Technology. Additional antibodies for phospho-ERK1,2 detection for western blot were from Promega (V803A), and Santa Cruz (E-Y, SC7383). Total ERK antibody for western blot analysis was K-23 from Santa Cruz (SC-94). Ki67 antibody (ab833) was from ABCAM, Mcl1 antibody (559027) was from BD Biosciences, Factor VIII antibody was from Dako (A082), CD31 antibody was from Dianova, (DIA310), and Cot antibody was from Santa Cruz Biotechnology (sc-373677). For the cyclin D1 second antibody staining was with an Alexa Fluor 568 donkey anti-rabbit IgG (Invitrogen, A10042) (1:200 dilution). The pMEK1 fluorescence was developed using the Alexa Fluor 488 chicken anti-rabbit IgG second antibody (1:200 dilution).TUNEL staining kits were from Promega (G2350). Mouse Implant Studies: Biopsy tissues were delivered to research laboratory in ice-cold Dulbecco's Modified Eagle Medium (DMEM) buffer solution. As the tissue mass available from each biopsy was limited, we first passaged the biopsy tissue in Balb/c nu/Foxn1 athymic nude mice (6-8 weeks of age and weighing 22-25g, purchased from Harlan Sprague Dawley, USA) to increase the volume of tumor for further implantation. -

Comparative Transcriptomics Reveals Similarities and Differences
Seifert et al. BMC Cancer (2015) 15:952 DOI 10.1186/s12885-015-1939-9 RESEARCH ARTICLE Open Access Comparative transcriptomics reveals similarities and differences between astrocytoma grades Michael Seifert1,2,5*, Martin Garbe1, Betty Friedrich1,3, Michel Mittelbronn4 and Barbara Klink5,6,7 Abstract Background: Astrocytomas are the most common primary brain tumors distinguished into four histological grades. Molecular analyses of individual astrocytoma grades have revealed detailed insights into genetic, transcriptomic and epigenetic alterations. This provides an excellent basis to identify similarities and differences between astrocytoma grades. Methods: We utilized public omics data of all four astrocytoma grades focusing on pilocytic astrocytomas (PA I), diffuse astrocytomas (AS II), anaplastic astrocytomas (AS III) and glioblastomas (GBM IV) to identify similarities and differences using well-established bioinformatics and systems biology approaches. We further validated the expression and localization of Ang2 involved in angiogenesis using immunohistochemistry. Results: Our analyses show similarities and differences between astrocytoma grades at the level of individual genes, signaling pathways and regulatory networks. We identified many differentially expressed genes that were either exclusively observed in a specific astrocytoma grade or commonly affected in specific subsets of astrocytoma grades in comparison to normal brain. Further, the number of differentially expressed genes generally increased with the astrocytoma grade with one major exception. The cytokine receptor pathway showed nearly the same number of differentially expressed genes in PA I and GBM IV and was further characterized by a significant overlap of commonly altered genes and an exclusive enrichment of overexpressed cancer genes in GBM IV. Additional analyses revealed a strong exclusive overexpression of CX3CL1 (fractalkine) and its receptor CX3CR1 in PA I possibly contributing to the absence of invasive growth. -
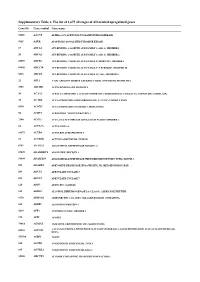
Supplementary Table 1. the List of 1,675 All Stages of AD-Related Upregulated Genes
Supplementary Table 1. The list of 1,675 all stages of AD-related upregulated genes Gene ID Gene symbol Gene name 51146 A4GNT ALPHA-1,4-N-ACETYLGLUCOSAMINYLTRANSFERASE 9625 AATK APOPTOSIS-ASSOCIATED TYROSINE KINASE 19 ABCA1 ATP-BINDING CASSETTE, SUB-FAMILY A (ABC1), MEMBER 1 20 ABCA2 ATP-BINDING CASSETTE, SUB-FAMILY A (ABC1), MEMBER 2 10058 ABCB6 ATP-BINDING CASSETTE, SUB-FAMILY B (MDR/TAP), MEMBER 6 89845 ABCC10 ATP-BINDING CASSETTE, SUB-FAMILY C (CFTR/MRP), MEMBER 10 5826 ABCD4 ATP-BINDING CASSETTE, SUB-FAMILY D (ALD), MEMBER 4 25 ABL1 V-ABL ABELSON MURINE LEUKEMIA VIRAL ONCOGENE HOMOLOG 1 3983 ABLIM1 ACTIN BINDING LIM PROTEIN 1 30 ACAA1 ACETYL-COENZYME A ACYLTRANSFERASE 1 (PEROXISOMAL 3-OXOACYL-COENZYME A THIOLASE) 35 ACADS ACYL-COENZYME A DEHYDROGENASE, C-2 TO C-3 SHORT CHAIN 8310 ACOX3 ACYL-COENZYME A OXIDASE 3, PRISTANOYL 56 ACRV1 ACROSOMAL VESICLE PROTEIN 1 2180 ACSL1 ACYL-COA SYNTHETASE LONG-CHAIN FAMILY MEMBER 1 86 ACTL6A ACTIN-LIKE 6A 93973 ACTR8 ACTIN-RELATED PROTEIN 8 91 ACVR1B ACTIVIN A RECEPTOR, TYPE IB 8747 ADAM21 ADAM METALLOPEPTIDASE DOMAIN 21 27299 ADAMDEC1 ADAM-LIKE, DECYSIN 1 56999 ADAMTS9 ADAM METALLOPEPTIDASE WITH THROMBOSPONDIN TYPE 1 MOTIF, 9 105 ADARB2 ADENOSINE DEAMINASE, RNA-SPECIFIC, B2 (RED2 HOMOLOG RAT) 109 ADCY3 ADENYLATE CYCLASE 3 113 ADCY7 ADENYLATE CYCLASE 7 120 ADD3 ADDUCIN 3 (GAMMA) 125 ADH1C ALCOHOL DEHYDROGENASE 1A (CLASS I), ALPHA POLYPEPTIDE 9370 ADIPOQ ADIPONECTIN, C1Q AND COLLAGEN DOMAIN CONTAINING 165 AEBP1 AE BINDING PROTEIN 1 4299 AFF1 AF4/FMR2 FAMILY, MEMBER 1 173 AFM AFAMIN 79814 AGMAT AGMATINE -

The Transcriptional and Epigenetic Role of Brd4 in the Regulation of the Cellular Stress Response
THE TRANSCRIPTIONAL AND EPIGENETIC ROLE OF BRD4 IN THE REGULATION OF THE CELLULAR STRESS RESPONSE INAUGURAL-DISSERTATION to obtain the academic degree Doctor rerum naturalium (Dr. rer. nat.) submitted to the Department of Biology, Chemistry and Pharmacy of Freie Universität Berlin by Michelle Hussong from Zweibrücken 2015 Die vorliegende Arbeit wurde im Zeitraum von Juli 2012 bis September 2015 am Max- Planck-Institut für Molekulare Genetik in Berlin sowie an der Universität zu Köln unter der Leitung von Frau Prof. Dr. Dr. Michal-Ruth Schweiger angefertigt. 1. Gutachter: Prof. Dr. Dr. Michal-Ruth Schweiger 2. Gutachter: Prof. Dr. Rupert Mutzel Disputation am 07.12.2015 ACKNOWLEDGMENT ACKNOWLEDGMENT This dissertation would not have been possible without the guidance and the help of many people who in one way or another contributed to the preparation and completion of this study. Firstly, I would like to express my sincere gratitude to my advisor Prof. Dr. Dr. Michal-Ruth Schweiger, for her continuous support throughout my PhD study, for her patience, motivation, and immense knowledge. I am eminently thankful for the multiple possibilities she gave me to work on this interesting and challenging field of research. I also want to thank Professor Dr. Rupert Mutzel for taking the time of being my second supervisor. My sincere thanks also goes to Prof. Dr. Hans Lehrach for having given me the opportunity to do my PhD thesis in the extraordinary and inspiring environment at the Max-Planck- Institute for Molecular Genetics in Berlin. Especially, the multitude of technologies and knowledge in his department made my work successful. -

The DNA Sequence and Comparative Analysis of Human Chromosome 20
articles The DNA sequence and comparative analysis of human chromosome 20 P. Deloukas, L. H. Matthews, J. Ashurst, J. Burton, J. G. R. Gilbert, M. Jones, G. Stavrides, J. P. Almeida, A. K. Babbage, C. L. Bagguley, J. Bailey, K. F. Barlow, K. N. Bates, L. M. Beard, D. M. Beare, O. P. Beasley, C. P. Bird, S. E. Blakey, A. M. Bridgeman, A. J. Brown, D. Buck, W. Burrill, A. P. Butler, C. Carder, N. P. Carter, J. C. Chapman, M. Clamp, G. Clark, L. N. Clark, S. Y. Clark, C. M. Clee, S. Clegg, V. E. Cobley, R. E. Collier, R. Connor, N. R. Corby, A. Coulson, G. J. Coville, R. Deadman, P. Dhami, M. Dunn, A. G. Ellington, J. A. Frankland, A. Fraser, L. French, P. Garner, D. V. Grafham, C. Grif®ths, M. N. D. Grif®ths, R. Gwilliam, R. E. Hall, S. Hammond, J. L. Harley, P. D. Heath, S. Ho, J. L. Holden, P. J. Howden, E. Huckle, A. R. Hunt, S. E. Hunt, K. Jekosch, C. M. Johnson, D. Johnson, M. P. Kay, A. M. Kimberley, A. King, A. Knights, G. K. Laird, S. Lawlor, M. H. Lehvaslaiho, M. Leversha, C. Lloyd, D. M. Lloyd, J. D. Lovell, V. L. Marsh, S. L. Martin, L. J. McConnachie, K. McLay, A. A. McMurray, S. Milne, D. Mistry, M. J. F. Moore, J. C. Mullikin, T. Nickerson, K. Oliver, A. Parker, R. Patel, T. A. V. Pearce, A. I. Peck, B. J. C. T. Phillimore, S. R. Prathalingam, R. W. Plumb, H. Ramsay, C. M.