An Integrative Systems Biology Approach Identifies Molecular
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

BIRC7 Sirna (Human)
For research purposes only, not for human use Product Data Sheet BIRC7 siRNA (Human) Catalog # Source Reactivity Applications CRJ2387 Synthetic H RNAi Description siRNA to inhibit BIRC7 expression using RNA interference Specificity BIRC7 siRNA (Human) is a target-specific 19-23 nt siRNA oligo duplexes designed to knock down gene expression. Form Lyophilized powder Gene Symbol BIRC7 Alternative Names KIAP; LIVIN; MLIAP; RNF50; Baculoviral IAP repeat-containing protein 7; Kidney inhibitor of apoptosis protein; KIAP; Livin; Melanoma inhibitor of apoptosis protein; ML-IAP; RING finger protein 50 Entrez Gene 79444 (Human) SwissProt Q96CA5 (Human) Purity > 97% Quality Control Oligonucleotide synthesis is monitored base by base through trityl analysis to ensure appropriate coupling efficiency. The oligo is subsequently purified by affinity-solid phase extraction. The annealed RNA duplex is further analyzed by mass spectrometry to verify the exact composition of the duplex. Each lot is compared to the previous lot by mass spectrometry to ensure maximum lot-to-lot consistency. Components We offers pre-designed sets of 3 different target-specific siRNA oligo duplexes of human BIRC7 gene. Each vial contains 5 nmol of lyophilized siRNA. The duplexes can be transfected individually or pooled together to achieve knockdown of the target gene, which is most commonly assessed by qPCR or western blot. Our siRNA oligos are also chemically modified (2’-OMe) at no extra charge for increased stability and Application key: E- ELISA, WB- Western blot, IH- Immunohistochemistry, -

Genome Wide Association Study of Response to Interval and Continuous Exercise Training: the Predict‑HIIT Study Camilla J
Williams et al. J Biomed Sci (2021) 28:37 https://doi.org/10.1186/s12929-021-00733-7 RESEARCH Open Access Genome wide association study of response to interval and continuous exercise training: the Predict-HIIT study Camilla J. Williams1†, Zhixiu Li2†, Nicholas Harvey3,4†, Rodney A. Lea4, Brendon J. Gurd5, Jacob T. Bonafglia5, Ioannis Papadimitriou6, Macsue Jacques6, Ilaria Croci1,7,20, Dorthe Stensvold7, Ulrik Wislof1,7, Jenna L. Taylor1, Trishan Gajanand1, Emily R. Cox1, Joyce S. Ramos1,8, Robert G. Fassett1, Jonathan P. Little9, Monique E. Francois9, Christopher M. Hearon Jr10, Satyam Sarma10, Sylvan L. J. E. Janssen10,11, Emeline M. Van Craenenbroeck12, Paul Beckers12, Véronique A. Cornelissen13, Erin J. Howden14, Shelley E. Keating1, Xu Yan6,15, David J. Bishop6,16, Anja Bye7,17, Larisa M. Haupt4, Lyn R. Grifths4, Kevin J. Ashton3, Matthew A. Brown18, Luciana Torquati19, Nir Eynon6 and Jef S. Coombes1* Abstract Background: Low cardiorespiratory ftness (V̇O2peak) is highly associated with chronic disease and mortality from all causes. Whilst exercise training is recommended in health guidelines to improve V̇O2peak, there is considerable inter-individual variability in the V̇O2peak response to the same dose of exercise. Understanding how genetic factors contribute to V̇O2peak training response may improve personalisation of exercise programs. The aim of this study was to identify genetic variants that are associated with the magnitude of V̇O2peak response following exercise training. Methods: Participant change in objectively measured V̇O2peak from 18 diferent interventions was obtained from a multi-centre study (Predict-HIIT). A genome-wide association study was completed (n 507), and a polygenic predictor score (PPS) was developed using alleles from single nucleotide polymorphisms= (SNPs) signifcantly associ- –5 ated (P < 1 10 ) with the magnitude of V̇O2peak response. -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

XIAP's Profile in Human Cancer
biomolecules Review XIAP’s Profile in Human Cancer Huailu Tu and Max Costa * Department of Environmental Medicine, Grossman School of Medicine, New York University, New York, NY 10010, USA; [email protected] * Correspondence: [email protected] Received: 16 September 2020; Accepted: 25 October 2020; Published: 29 October 2020 Abstract: XIAP, the X-linked inhibitor of apoptosis protein, regulates cell death signaling pathways through binding and inhibiting caspases. Mounting experimental research associated with XIAP has shown it to be a master regulator of cell death not only in apoptosis, but also in autophagy and necroptosis. As a vital decider on cell survival, XIAP is involved in the regulation of cancer initiation, promotion and progression. XIAP up-regulation occurs in many human diseases, resulting in a series of undesired effects such as raising the cellular tolerance to genetic lesions, inflammation and cytotoxicity. Hence, anti-tumor drugs targeting XIAP have become an important focus for cancer therapy research. RNA–XIAP interaction is a focus, which has enriched the general profile of XIAP regulation in human cancer. In this review, the basic functions of XIAP, its regulatory role in cancer, anti-XIAP drugs and recent findings about RNA–XIAP interactions are discussed. Keywords: XIAP; apoptosis; cancer; therapeutics; non-coding RNA 1. Introduction X-linked inhibitor of apoptosis protein (XIAP), also known as inhibitor of apoptosis protein 3 (IAP3), baculoviral IAP repeat-containing protein 4 (BIRC4), and human IAPs like protein (hILP), belongs to IAP family which was discovered in insect baculovirus [1]. Eight different IAPs have been isolated from human tissues: NAIP (BIRC1), BIRC2 (cIAP1), BIRC3 (cIAP2), XIAP (BIRC4), BIRC5 (survivin), BIRC6 (apollon), BIRC7 (livin) and BIRC8 [2]. -

Whole Exome Sequencing in Families at High Risk for Hodgkin Lymphoma: Identification of a Predisposing Mutation in the KDR Gene
Hodgkin Lymphoma SUPPLEMENTARY APPENDIX Whole exome sequencing in families at high risk for Hodgkin lymphoma: identification of a predisposing mutation in the KDR gene Melissa Rotunno, 1 Mary L. McMaster, 1 Joseph Boland, 2 Sara Bass, 2 Xijun Zhang, 2 Laurie Burdett, 2 Belynda Hicks, 2 Sarangan Ravichandran, 3 Brian T. Luke, 3 Meredith Yeager, 2 Laura Fontaine, 4 Paula L. Hyland, 1 Alisa M. Goldstein, 1 NCI DCEG Cancer Sequencing Working Group, NCI DCEG Cancer Genomics Research Laboratory, Stephen J. Chanock, 5 Neil E. Caporaso, 1 Margaret A. Tucker, 6 and Lynn R. Goldin 1 1Genetic Epidemiology Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, NIH, Bethesda, MD; 2Cancer Genomics Research Laboratory, Division of Cancer Epidemiology and Genetics, National Cancer Institute, NIH, Bethesda, MD; 3Ad - vanced Biomedical Computing Center, Leidos Biomedical Research Inc.; Frederick National Laboratory for Cancer Research, Frederick, MD; 4Westat, Inc., Rockville MD; 5Division of Cancer Epidemiology and Genetics, National Cancer Institute, NIH, Bethesda, MD; and 6Human Genetics Program, Division of Cancer Epidemiology and Genetics, National Cancer Institute, NIH, Bethesda, MD, USA ©2016 Ferrata Storti Foundation. This is an open-access paper. doi:10.3324/haematol.2015.135475 Received: August 19, 2015. Accepted: January 7, 2016. Pre-published: June 13, 2016. Correspondence: [email protected] Supplemental Author Information: NCI DCEG Cancer Sequencing Working Group: Mark H. Greene, Allan Hildesheim, Nan Hu, Maria Theresa Landi, Jennifer Loud, Phuong Mai, Lisa Mirabello, Lindsay Morton, Dilys Parry, Anand Pathak, Douglas R. Stewart, Philip R. Taylor, Geoffrey S. Tobias, Xiaohong R. Yang, Guoqin Yu NCI DCEG Cancer Genomics Research Laboratory: Salma Chowdhury, Michael Cullen, Casey Dagnall, Herbert Higson, Amy A. -

Chemical Agent and Antibodies B-Raf Inhibitor RAF265
Supplemental Materials and Methods: Chemical agent and antibodies B-Raf inhibitor RAF265 [5-(2-(5-(trifluromethyl)-1H-imidazol-2-yl)pyridin-4-yloxy)-N-(4-trifluoromethyl)phenyl-1-methyl-1H-benzp{D, }imidazol-2- amine] was kindly provided by Novartis Pharma AG and dissolved in solvent ethanol:propylene glycol:2.5% tween-80 (percentage 6:23:71) for oral delivery to mice by gavage. Antibodies to phospho-ERK1/2 Thr202/Tyr204(4370), phosphoMEK1/2(2338 and 9121)), phospho-cyclin D1(3300), cyclin D1 (2978), PLK1 (4513) BIM (2933), BAX (2772), BCL2 (2876) were from Cell Signaling Technology. Additional antibodies for phospho-ERK1,2 detection for western blot were from Promega (V803A), and Santa Cruz (E-Y, SC7383). Total ERK antibody for western blot analysis was K-23 from Santa Cruz (SC-94). Ki67 antibody (ab833) was from ABCAM, Mcl1 antibody (559027) was from BD Biosciences, Factor VIII antibody was from Dako (A082), CD31 antibody was from Dianova, (DIA310), and Cot antibody was from Santa Cruz Biotechnology (sc-373677). For the cyclin D1 second antibody staining was with an Alexa Fluor 568 donkey anti-rabbit IgG (Invitrogen, A10042) (1:200 dilution). The pMEK1 fluorescence was developed using the Alexa Fluor 488 chicken anti-rabbit IgG second antibody (1:200 dilution).TUNEL staining kits were from Promega (G2350). Mouse Implant Studies: Biopsy tissues were delivered to research laboratory in ice-cold Dulbecco's Modified Eagle Medium (DMEM) buffer solution. As the tissue mass available from each biopsy was limited, we first passaged the biopsy tissue in Balb/c nu/Foxn1 athymic nude mice (6-8 weeks of age and weighing 22-25g, purchased from Harlan Sprague Dawley, USA) to increase the volume of tumor for further implantation. -

Comparative Transcriptomics Reveals Similarities and Differences
Seifert et al. BMC Cancer (2015) 15:952 DOI 10.1186/s12885-015-1939-9 RESEARCH ARTICLE Open Access Comparative transcriptomics reveals similarities and differences between astrocytoma grades Michael Seifert1,2,5*, Martin Garbe1, Betty Friedrich1,3, Michel Mittelbronn4 and Barbara Klink5,6,7 Abstract Background: Astrocytomas are the most common primary brain tumors distinguished into four histological grades. Molecular analyses of individual astrocytoma grades have revealed detailed insights into genetic, transcriptomic and epigenetic alterations. This provides an excellent basis to identify similarities and differences between astrocytoma grades. Methods: We utilized public omics data of all four astrocytoma grades focusing on pilocytic astrocytomas (PA I), diffuse astrocytomas (AS II), anaplastic astrocytomas (AS III) and glioblastomas (GBM IV) to identify similarities and differences using well-established bioinformatics and systems biology approaches. We further validated the expression and localization of Ang2 involved in angiogenesis using immunohistochemistry. Results: Our analyses show similarities and differences between astrocytoma grades at the level of individual genes, signaling pathways and regulatory networks. We identified many differentially expressed genes that were either exclusively observed in a specific astrocytoma grade or commonly affected in specific subsets of astrocytoma grades in comparison to normal brain. Further, the number of differentially expressed genes generally increased with the astrocytoma grade with one major exception. The cytokine receptor pathway showed nearly the same number of differentially expressed genes in PA I and GBM IV and was further characterized by a significant overlap of commonly altered genes and an exclusive enrichment of overexpressed cancer genes in GBM IV. Additional analyses revealed a strong exclusive overexpression of CX3CL1 (fractalkine) and its receptor CX3CR1 in PA I possibly contributing to the absence of invasive growth. -
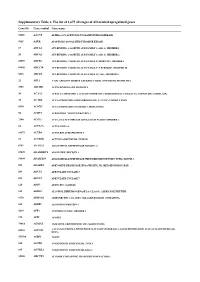
Supplementary Table 1. the List of 1,675 All Stages of AD-Related Upregulated Genes
Supplementary Table 1. The list of 1,675 all stages of AD-related upregulated genes Gene ID Gene symbol Gene name 51146 A4GNT ALPHA-1,4-N-ACETYLGLUCOSAMINYLTRANSFERASE 9625 AATK APOPTOSIS-ASSOCIATED TYROSINE KINASE 19 ABCA1 ATP-BINDING CASSETTE, SUB-FAMILY A (ABC1), MEMBER 1 20 ABCA2 ATP-BINDING CASSETTE, SUB-FAMILY A (ABC1), MEMBER 2 10058 ABCB6 ATP-BINDING CASSETTE, SUB-FAMILY B (MDR/TAP), MEMBER 6 89845 ABCC10 ATP-BINDING CASSETTE, SUB-FAMILY C (CFTR/MRP), MEMBER 10 5826 ABCD4 ATP-BINDING CASSETTE, SUB-FAMILY D (ALD), MEMBER 4 25 ABL1 V-ABL ABELSON MURINE LEUKEMIA VIRAL ONCOGENE HOMOLOG 1 3983 ABLIM1 ACTIN BINDING LIM PROTEIN 1 30 ACAA1 ACETYL-COENZYME A ACYLTRANSFERASE 1 (PEROXISOMAL 3-OXOACYL-COENZYME A THIOLASE) 35 ACADS ACYL-COENZYME A DEHYDROGENASE, C-2 TO C-3 SHORT CHAIN 8310 ACOX3 ACYL-COENZYME A OXIDASE 3, PRISTANOYL 56 ACRV1 ACROSOMAL VESICLE PROTEIN 1 2180 ACSL1 ACYL-COA SYNTHETASE LONG-CHAIN FAMILY MEMBER 1 86 ACTL6A ACTIN-LIKE 6A 93973 ACTR8 ACTIN-RELATED PROTEIN 8 91 ACVR1B ACTIVIN A RECEPTOR, TYPE IB 8747 ADAM21 ADAM METALLOPEPTIDASE DOMAIN 21 27299 ADAMDEC1 ADAM-LIKE, DECYSIN 1 56999 ADAMTS9 ADAM METALLOPEPTIDASE WITH THROMBOSPONDIN TYPE 1 MOTIF, 9 105 ADARB2 ADENOSINE DEAMINASE, RNA-SPECIFIC, B2 (RED2 HOMOLOG RAT) 109 ADCY3 ADENYLATE CYCLASE 3 113 ADCY7 ADENYLATE CYCLASE 7 120 ADD3 ADDUCIN 3 (GAMMA) 125 ADH1C ALCOHOL DEHYDROGENASE 1A (CLASS I), ALPHA POLYPEPTIDE 9370 ADIPOQ ADIPONECTIN, C1Q AND COLLAGEN DOMAIN CONTAINING 165 AEBP1 AE BINDING PROTEIN 1 4299 AFF1 AF4/FMR2 FAMILY, MEMBER 1 173 AFM AFAMIN 79814 AGMAT AGMATINE -

Microphthalmia-Associated Transcription Factor Controls the DNA Damage Response and a Lineage-Specific Senescence Program in Melanomas
Published OnlineFirst April 13, 2010; DOI: 10.1158/0008-5472.CAN-09-2913 Tumor and Stem Cell Biology Cancer Research Microphthalmia-Associated Transcription Factor Controls the DNA Damage Response and a Lineage-Specific Senescence Program in Melanomas Sandy Giuliano1,2, Yann Cheli1,2, Mickaël Ohanna1,2, Caroline Bonet1,2, Laurent Beuret1,2, Karine Bille1,2, Agnès Loubat2,4, Véronique Hofman2,5, Paul Hofman2,5, Gilles Ponzio2,4, Philippe Bahadoran3, Robert Ballotti1,2, and Corine Bertolotto1,2 Abstract Apoptosis and senescence are cellular failsafe programs that counteract excessive mitogenic signaling ob- served in cancer cells. Melanoma is known for its notorious resistance to apoptotic processes; therefore, se- nescence, which remains poorly understood in melanomas, can be viewed as a therapeutic alternative. Microphthalmia-associated transcription factor (MITF), in which its M transcript is specifically expressed in melanocyte cells, plays a critical role in melanoma proliferation, and its specific inhibition is associated with G0-G1 growth arrest. Interestingly, decreased MITF expression has been described in senescent melano- cytes, and we have observed an inhibition of MITF expression in melanoma cells exposed to chemotherapeutic drugs that induce their senescence. All these observations thereby question the role of MITF in controlling senescence in melanoma cells. Here, we report that long-term depletion of MITF in melanoma cells triggers a senescence program characterized by typical morphologic and biochemical changes associated with a sus- tained growth arrest. Further, we show that MITF-silenced cells engage a DNA damage response (DDR) sig- naling pathway, leading to p53 upregulation, which is critically required for senescence entry. This study uncovers the existence of a lineage-restricted DDR/p53 signaling pathway that is inhibited by MITF to prevent senescence and favor melanoma cell proliferation. -

Gene Expression in the Mouse Eye: an Online Resource for Genetics Using 103 Strains of Mice
Molecular Vision 2009; 15:1730-1763 <http://www.molvis.org/molvis/v15/a185> © 2009 Molecular Vision Received 3 September 2008 | Accepted 25 August 2009 | Published 31 August 2009 Gene expression in the mouse eye: an online resource for genetics using 103 strains of mice Eldon E. Geisert,1 Lu Lu,2 Natalie E. Freeman-Anderson,1 Justin P. Templeton,1 Mohamed Nassr,1 Xusheng Wang,2 Weikuan Gu,3 Yan Jiao,3 Robert W. Williams2 (First two authors contributed equally to this work) 1Department of Ophthalmology and Center for Vision Research, Memphis, TN; 2Department of Anatomy and Neurobiology and Center for Integrative and Translational Genomics, Memphis, TN; 3Department of Orthopedics, University of Tennessee Health Science Center, Memphis, TN Purpose: Individual differences in patterns of gene expression account for much of the diversity of ocular phenotypes and variation in disease risk. We examined the causes of expression differences, and in their linkage to sequence variants, functional differences, and ocular pathophysiology. Methods: mRNAs from young adult eyes were hybridized to oligomer microarrays (Affymetrix M430v2). Data were embedded in GeneNetwork with millions of single nucleotide polymorphisms, custom array annotation, and information on complementary cellular, functional, and behavioral traits. The data include male and female samples from 28 common strains, 68 BXD recombinant inbred lines, as well as several mutants and knockouts. Results: We provide a fully integrated resource to map, graph, analyze, and test causes and correlations of differences in gene expression in the eye. Covariance in mRNA expression can be used to infer gene function, extract signatures for different cells or tissues, to define molecular networks, and to map quantitative trait loci that produce expression differences. -

The DNA Sequence and Comparative Analysis of Human Chromosome 20
articles The DNA sequence and comparative analysis of human chromosome 20 P. Deloukas, L. H. Matthews, J. Ashurst, J. Burton, J. G. R. Gilbert, M. Jones, G. Stavrides, J. P. Almeida, A. K. Babbage, C. L. Bagguley, J. Bailey, K. F. Barlow, K. N. Bates, L. M. Beard, D. M. Beare, O. P. Beasley, C. P. Bird, S. E. Blakey, A. M. Bridgeman, A. J. Brown, D. Buck, W. Burrill, A. P. Butler, C. Carder, N. P. Carter, J. C. Chapman, M. Clamp, G. Clark, L. N. Clark, S. Y. Clark, C. M. Clee, S. Clegg, V. E. Cobley, R. E. Collier, R. Connor, N. R. Corby, A. Coulson, G. J. Coville, R. Deadman, P. Dhami, M. Dunn, A. G. Ellington, J. A. Frankland, A. Fraser, L. French, P. Garner, D. V. Grafham, C. Grif®ths, M. N. D. Grif®ths, R. Gwilliam, R. E. Hall, S. Hammond, J. L. Harley, P. D. Heath, S. Ho, J. L. Holden, P. J. Howden, E. Huckle, A. R. Hunt, S. E. Hunt, K. Jekosch, C. M. Johnson, D. Johnson, M. P. Kay, A. M. Kimberley, A. King, A. Knights, G. K. Laird, S. Lawlor, M. H. Lehvaslaiho, M. Leversha, C. Lloyd, D. M. Lloyd, J. D. Lovell, V. L. Marsh, S. L. Martin, L. J. McConnachie, K. McLay, A. A. McMurray, S. Milne, D. Mistry, M. J. F. Moore, J. C. Mullikin, T. Nickerson, K. Oliver, A. Parker, R. Patel, T. A. V. Pearce, A. I. Peck, B. J. C. T. Phillimore, S. R. Prathalingam, R. W. Plumb, H. Ramsay, C. M. -

Mouse Birc7 Knockout Project (CRISPR/Cas9)
https://www.alphaknockout.com Mouse Birc7 Knockout Project (CRISPR/Cas9) Objective: To create a Birc7 knockout Mouse model (C57BL/6J) by CRISPR/Cas-mediated genome engineering. Strategy summary: The Birc7 gene (NCBI Reference Sequence: NM_001163247 ; Ensembl: ENSMUSG00000038840 ) is located on Mouse chromosome 2. 7 exons are identified, with the ATG start codon in exon 1 and the TAA stop codon in exon 6 (Transcript: ENSMUST00000108875). Exon 1~6 will be selected as target site. Cas9 and gRNA will be co-injected into fertilized eggs for KO Mouse production. The pups will be genotyped by PCR followed by sequencing analysis. Note: Mice homozygous for a knock-out allele exhibit normal retinal morphology and function. Exon 1 starts from about 0.12% of the coding region. Exon 1~6 covers 100.0% of the coding region. The size of effective KO region: ~4181 bp. The KO region does not have any other known gene. Page 1 of 8 https://www.alphaknockout.com Overview of the Targeting Strategy Wildtype allele 5' gRNA region gRNA region 3' 1 2 3 4 5 6 7 Legends Exon of mouse Birc7 Knockout region Page 2 of 8 https://www.alphaknockout.com Overview of the Dot Plot (up) Window size: 15 bp Forward Reverse Complement Sequence 12 Note: The 2000 bp section upstream of start codon is aligned with itself to determine if there are tandem repeats. Tandem repeats are found in the dot plot matrix. The gRNA site is selected outside of these tandem repeats. Overview of the Dot Plot (down) Window size: 15 bp Forward Reverse Complement Sequence 12 Note: The 2000 bp section downstream of stop codon is aligned with itself to determine if there are tandem repeats.