Primitive Streak Stage (18 Hr) X- S
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Coronary Arterial Development Is Regulated by a Dll4-Jag1-Ephrinb2 Signaling Cascade
RESEARCH ARTICLE Coronary arterial development is regulated by a Dll4-Jag1-EphrinB2 signaling cascade Stanislao Igor Travisano1,2, Vera Lucia Oliveira1,2, Bele´ n Prados1,2, Joaquim Grego-Bessa1,2, Rebeca Pin˜ eiro-Sabarı´s1,2, Vanesa Bou1,2, Manuel J Go´ mez3, Fa´ tima Sa´ nchez-Cabo3, Donal MacGrogan1,2*, Jose´ Luis de la Pompa1,2* 1Intercellular Signalling in Cardiovascular Development and Disease Laboratory, Centro Nacional de Investigaciones Cardiovasculares Carlos III (CNIC), Madrid, Spain; 2CIBER de Enfermedades Cardiovasculares, Madrid, Spain; 3Bioinformatics Unit, Centro Nacional de Investigaciones Cardiovasculares, Madrid, Spain Abstract Coronaries are essential for myocardial growth and heart function. Notch is crucial for mouse embryonic angiogenesis, but its role in coronary development remains uncertain. We show Jag1, Dll4 and activated Notch1 receptor expression in sinus venosus (SV) endocardium. Endocardial Jag1 removal blocks SV capillary sprouting, while Dll4 inactivation stimulates excessive capillary growth, suggesting that ligand antagonism regulates coronary primary plexus formation. Later endothelial ligand removal, or forced expression of Dll4 or the glycosyltransferase Mfng, blocks coronary plexus remodeling, arterial differentiation, and perivascular cell maturation. Endocardial deletion of Efnb2 phenocopies the coronary arterial defects of Notch mutants. Angiogenic rescue experiments in ventricular explants, or in primary human endothelial cells, indicate that EphrinB2 is a critical effector of antagonistic Dll4 and Jag1 functions in arterial morphogenesis. Thus, coronary arterial precursors are specified in the SV prior to primary coronary plexus formation and subsequent arterial differentiation depends on a Dll4-Jag1-EphrinB2 signaling *For correspondence: [email protected] (DMG); cascade. [email protected] (JLP) Competing interests: The authors declare that no Introduction competing interests exist. -

The Evolving Cardiac Lymphatic Vasculature in Development, Repair and Regeneration
REVIEWS The evolving cardiac lymphatic vasculature in development, repair and regeneration Konstantinos Klaourakis 1,2, Joaquim M. Vieira 1,2,3 ✉ and Paul R. Riley 1,2,3 ✉ Abstract | The lymphatic vasculature has an essential role in maintaining normal fluid balance in tissues and modulating the inflammatory response to injury or pathogens. Disruption of normal development or function of lymphatic vessels can have severe consequences. In the heart, reduced lymphatic function can lead to myocardial oedema and persistent inflammation. Macrophages, which are phagocytic cells of the innate immune system, contribute to cardiac development and to fibrotic repair and regeneration of cardiac tissue after myocardial infarction. In this Review, we discuss the cardiac lymphatic vasculature with a focus on developments over the past 5 years arising from the study of mammalian and zebrafish model organisms. In addition, we examine the interplay between the cardiac lymphatics and macrophages during fibrotic repair and regeneration after myocardial infarction. Finally, we discuss the therapeutic potential of targeting the cardiac lymphatic network to regulate immune cell content and alleviate inflammation in patients with ischaemic heart disease. The circulatory system of vertebrates is composed of two after MI. In this Review, we summarize the current complementary vasculatures, the blood and lymphatic knowledge on the development, structure and function vascular systems1. The blood vasculature is a closed sys- of the cardiac lymphatic vasculature, with an emphasis tem responsible for transporting gases, fluids, nutrients, on breakthroughs over the past 5 years in the study of metabolites and cells to the tissues2. This extravasation of cardiac lymphatic heterogeneity in mice and zebrafish. -

Cardiogenesis with a Focus on Vasculogenesis and Angiogenesis
Received: 27 August 2019 | Revised: 4 February 2020 | Accepted: 20 February 2020 DOI: 10.1111/ahe.12549 SPECIAL ISSUE Cardiogenesis with a focus on vasculogenesis and angiogenesis Katrin Borasch1 | Kenneth Richardson2 | Johanna Plendl1 1Department of Veterinary Medicine, Institute of Veterinary Anatomy, Freie Abstract University Berlin, Berlin, Germany The initial intraembryonic vasculogenesis occurs in the cardiogenic mesoderm. Here, 2 College of Veterinary Medicine, School a cell population of proendocardial cells detaches from the mesoderm that subse- of Veterinary and Life Sciences, Murdoch University, Murdoch, WA, Australia quently generates the single endocardial tube by forming vascular plexuses. In the course of embryogenesis, the endocardium retains vasculogenic, angiogenic and Correspondence Johanna Plendl, Department of Veterinary haematopoietic potential. The coronary blood vessels that sustain the rapidly ex- Medicine, Institute of Veterinary Anatomy, panding myocardium develop in the course of the formation of the cardiac loop by Freie University Berlin, Berlin, Germany. Email: [email protected] vasculogenesis and angiogenesis from progenitor cells of the proepicardial serosa at the venous pole of the heart as well as from the endocardium and endothelial cells of Funding information Freie Universität Berlin the sinus venosus. Prospective coronary endothelial cells and progenitor cells of the coronary blood vessel walls (smooth muscle cells, perivascular cells) originate from different cell populations that are in close spatial as well as regulatory connection with each other. Vasculo- and angiogenesis of the coronary blood vessels are for a large part regulated by the epicardium and epicardium-derived cells. Vasculogenic and angiogenic signalling pathways include the vascular endothelial growth factors, the angiopoietins and the fibroblast growth factors and their receptors. -

Cardiovascular System Note: the Cardiovascular System Develops Early (Week 3), Enabling the Embryo to Grow Beyond the Short
Lymphatics: Lymph vessel formation is similar to blood angiogenesis. Lymphatics begin as lymph sacs in three regions: jugular (near brachiocephalic veins); cranial abdominal (future cysterna chyla); and iliac region. Lym- phatic vessels (ducts) form as outgrowths of the sacs. mesenchyme Lymph nodes are produced by localized mesoder- sinusoid lymph duct lumen mal invaginations that partition the vessel lumen into sinu- soids. The mesoderm develops a reticular framework within which mesodermal lymphocytes accumulate. The spleen and hemal nodes (in ruminants) invagination develop similar to the way lymph nodes develop. Lymph Node Formation Prior to birth, fetal circulation is designed for an in utero aqueous environment where the pla- centa oxygenates fetal blood. Suddenly, at birth... Three In-Utero Adjustments ductus Stretching and constriction of arteriosus umbilical arteries shifts fetal blood flow aortic arch from the placenta to the fetus. Reduced pulmonary trunk L atrium venous return through the (left) umbili- foramen ovale R cal vein and ductus venosus allows the atrium latter to gradually close (over a period caudal vena cava of days). Bradykinin released by expand- ductus venosus ing lungs and increased oxygen concen- tration in blood triggers constriction of aorta the ductus arteriosus which, over two liver months, is gradually converted to a fibrous structure, the ligamentum arte- umbilical v. riosum. portal v. The increased blood flow to the lungs and then to the left atrium equalizes pres- sure in the two atria, resulting in closure umbilical aa. of the foramen ovale that eventually grows permanent. 29 The cardiogenic area, the place where the embryonic heart originates, is located . -

Cardiac Development Cardiac Development
CARDIAC DEVELOPMENT CARDIAC DEVELOPMENT Diane E. Spicer, BS, PA(ASCP) University of Florida Dept. of Pediatric Cardiology Curator – Van Mierop Cardiac Archive This lecture is given with special thanks to Professor RH Anderson, my mentor and my friend. Without his spectacular research and images of both human and mouse embryos, this lecture would not have been possible. CARDIAC DEVELOPMENT CARDIAC DEVELOPMENT ♥ What’s new? ♥ “An understanding of the elementary facts of human and comparative embryology is essential to an intelligent grasp of the ontogenetic problems of congenital cardiac disease.” ♥ Maude Abbott “Atlas of Congenital Cardiac Disease” American Heart Association, New York, 1936 CARDIACCARDIAC DEVELOPMENTDEVELOPMENT ♥ Do we need to change? ♥ In the past, most theories of morphogenesis were based on fanciful interpretation of normal development ♥ We are now able to demonstrate the anatomic and molecular changes that take place during cardiac development ♥ This now permits us to base our inferences on evidence, rather than speculation CARDIACCARDIAC DEVELOPMENTDEVELOPMENT ♥ It used to be thought that all components of the postnatal heart were contained within the initial linear heart tube ♥ In reality, new material is added at the arterial and venous poles from the second heart field. The initial tube, derived from the first heart field, forms little more than the definitive left ventricle Mouse embryo – 9 somites – Myosin LC Growth at arterial pole Putative left ventricle Growth at venous pole Mouse embryo – E8.5 – 9 somites -

The Interventricular Septum by E
Thorax: first published as 10.1136/thx.12.4.304 on 1 December 1957. Downloaded from Thorax (1957), 12, 304. THE INTERVENTRICULAR SEPTUM BY E. W. T. MORRIS From the Anatomy Department, St. Thomas's Hospital Medical School, Londoni (RECEIVED FOR PUBLICATION JULY 26, 1957) It is difficult to find in the literature a clear and between the tips of its two horns where the concise account of the development and form of boundary is formed by the fused atrioventricular the interventricular septum. Moreover, some of cushion (A, in Fig. 4). This septum does not lie the accounts in the clinical literature are at vari- in one plane and the main part of its free border ance with that generally accepted by embryo- forms a spiral (Figs. 4 and 5). logists. For this reason and in view of the recent (2) While the muscular part is forming. changes technical advances in the surgery of the heart, it are taking place in the relative positions of the seems opportune to describe the development and bulbus cordis and the ventricles. Earlier the heart anatomy of the interventricular septum and to tube is flexed at the bulboventricular junction so correlate this knowledge as far as possible with that the bulbus cordis comes to lie ventrally and the sites of interventricular septal defects. to the right of the ventricle (Fig. 2). Their con- At an early stage the heart consists of the sinus tiguous walls form a septum-the bulboven- venosus, the common atrium, the common ven- tricular septum-around the lower free border tricle, and the bulbus cordis, serially arranged in of which the two cavities communicate (see Figs.copyright. -
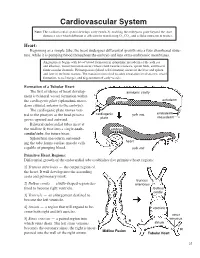
Cardiovascular System Note: the Cardiovascular System Develops Early (Week-3), Enabling the Embryo to Grow Beyond the Short
Cardiovascular System Note: The cardiovascular system develops early (week-3), enabling the embryo to grow beyond the short distances over which diffusion is efficient for transferring 2O , CO2, and cellular nutrients & wastes. Heart: Beginning as a simple tube, the heart undergoes differential growth into a four chambered struc- ture, while it is pumping blood throughout the embryo and into extra-embryonic membranes. Angiogenesis begins with blood island formation in splanchnic mesoderm of the yolk sac and allantois. Vessel formation occurs when island vesicles coalesce, sprout buds, and fuse to form vascular channels. Hematopoiesis (blood cell formation) occurs in the liver and spleen and later in the bone marrow. The transition from fetal to adult circulation involves new vessel formation, vessel merger, and degeneration of early vessels. Formation of a Tubular Heart: The first evidence of heart develop- amnionic cavity ment is bilateral vessel formation within ectoderm the cardiogenic plate (splanchnic meso- embryo derm situated anterior to the embryo). The cardiogenic plate moves ven- tral to the pharynx as the head process cardiogenic yolk sac endoderm mesoderm grows upward and outward. plate Bilateral endocardial tubes meet at the midline & fuse into a single endo- embryo cardial tube, the future heart. Splanchnic mesoderm surround- ing the tube forms cardiac muscle cells heart capable of pumping blood. yolk sac Primitive Heart Regions: Differential growth of the endocardial tube establishes five primitive heart regions: 1] Truncus arteriosus — the output region of the heart. It will develop into the ascending aorta and pulmonary trunk. truncus 2] Bulbus cordis — a bulb-shaped region des- arteriosus tined to become right ventricle. -

The Sinus Venosus Typeof Interatrial Septal Defect*
Thorax: first published as 10.1136/thx.13.1.12 on 1 March 1958. Downloaded from Thorax (I9%8), 13, 12. THE SINUS VENOSUS TYPE OF INTERATRIAL SEPTAL DEFECT* BY H. R. S. HARLEY Cardiff (RECEIVED FOR PUBLICATION DECEMBER 30, 1957) Defects of the interatrial septum, other than namely, (1) it lies above and independent of valvular patency of the foramen ovale, are often the fossa ovalis; (2) its margin is incomplete, classified into ostium primum and ostium secun- being absent superiorly and incomplete pos- dum varieties. The relationship of the former type teriorly; and (3) it is associated with anomalous to abnormal development of the atrioventricular drainage of the right superior, and sometimes of canal has been stressed by several workers, includ- the right middle or inferior, pulmonary vein. This ing Rogers and Edwards (1948), Wakai and type of defect is illustrated in Fig. 1 (after Lewis Edwards (1956), Wakai, Swan, and Wood (1956), et al., 1955) and Fig. 2 (after Geddes, 1912). In Brandenburg and DuShane (1956), Toscano- the case reported by Ross (1956), who kindly per- Barbosa, Brandenburg, and Burchell (1956), and mitted me to see the heart, the interatrial Cooley and Kirklin (1956). These workers prefer communication was described as ". lying the term "persistent common within the orifice of atrioventricular the superior vena cava in itscopyright. canal " to "persistent ostium primum." medial wall opposite the mouths of the anomalous In addition to the above types of interatrial pulmonary veins." Ross goes on to say: "On septal defect there is a third variety, which was casual inspection of the interior of the left atrium, described as long ago as 1868 by Wagstaffe, but the defect was not visible unless a search was made which has come into prominence only since the within the superior caval orifice." The relation- http://thorax.bmj.com/ introduction of surgical repair of interatrial ship of the defect to the orifice of the superior communications under direct vision. -

A Very Small Sinus Venosus Type of Atrial Septal Defect: a Rare but Curable Cause of Recurrent Stroke
Neurology Asia 2015; 20(3) : 283 – 285 CASE REPORTS A very small sinus venosus type of atrial septal defect: A rare but curable cause of recurrent stroke 1Seongheon Kim MD, 2Sung-Min Park MD, 2Se-Min Ryu MD, 3Dong Ryeol Ryu MD 1Department of Neurology, 2Department of Thoracic Surgery, 3Division of Cardiology, Department of Internal Medicine, Kangwon National University Hospital, Chuncheon, Korea Abstract Sinus venosus is a rare cardiac defect, which may lead to an interatrial shunt. Diagnosis on echocardiography may be difficult requiring an evaluation by a board-certified cardiologist. We report a case of a 41 year-old male who presented with recurrent episodes of hemiparesis (first left sided, second right sided). Surgical correction of sinus venosus led to resolution of his symptoms. INTRODUCTION coagulation parameters were within normal limits. Antithrombin3 was 86.5%, lupus anticoagulant Atrial septal defects close to the superior vena was negative, protein S was 60%, and protein C cava are called sinus venosus atrial septal was 97%. Peripheral blood smear was normal. defects (ASD). They often include apparent or Values for antinuclear antibody, double-stranded true anomalous entry of one or both of the right DNA antibodies, ANCA and ACA IgG were all pulmonary veins into the right superior vena within normal limits. cava or the right atrium. Thus, this malformation Electrocardiography showed normal sinus can cause interatrial shunting leading to rhythm. 24-hour Holter monitoring was rightventricular overload. Clinical manifestations unremarkable. Transthoracic echocardiogram range from mild to severe in degree, with most demonstrated left atrial enlargement and minimal patients having minimal symptoms. -

Dr. Kumari Sushma Saroj Department: Zoology College: Dr
1 Faculty Name: Dr. Kumari Sushma Saroj Department: Zoology College: Dr. L. K. V. D College, Tajpur, Samastipur Class: B.Sc Part- I (Sub.) Group: B Topic: Circulatory system of Scoliodon Circulatory System of Scoliodon Kingdom: Animalia Phylum: Chordata Class: Chondrichthyes Oder: Carcharhiniformes Family: Carcharhinidae Genus: Scoliodon Majority of multicellular animal possess a well developed circulatory system or cardiovascular system. “Cardiovascular” comes from the Greek, Kardia (heart) and Latin, vasculum (vessel). Though the circulatory system varies in different animal groups, it serves the following principal functions: Nutrients and waste products transport Oxygen and carbon dioxide transport Carries metabolic intermediates Hormone transport to target tissue Uniform distribution of water, H+, chemical substances and body heat. The circulatory system of Scoliodon is well developed and comprises of 4 parts – Heart and Pericardium, Arteries, Veins and blood. 1. Heart and Pericardium: Dr. Kumari Sushma Saroj, Dept. of Zoology, Dr. L.K.V.D. college, Tajpur, Samastipur 2 Similar to cyclostomes and other fishes, the heart of Scoliodon receives venous blood only which it pumps into gills for aeration. Such a heart is called venous or branchial heart. It is situated mid-ventrally in head beneath the pharynx supported below by the coracoid cartilages of the pectoral girdle. It lies within the pericardial cavity of two layered membranous sacs the pericardium. Heart is a reddish-brown, muscular and dorso-ventrally bent, S- shaped tube differentiated into a series of 4 chambers; sinus venosus, auricle, ventricle and conus arteriosus. .. But only two auricles and ventricle are considered true chambers, so that heart is only two chambers. -

6. Heart and Circulatory System I
6. HEART AND CIRCULATORY SYSTEM I Dr. Taube P. Rothman P&S 12-520 [email protected] 212-305-7930 RECOMMENDED READING: Larsen Human Embryology, 3rd Edition, pp. 195-199; 157-169 top left; 172-174; bottom 181-182; 187-top 189, Simbryo-cardiovascular system SUMMARY: The circulatory system, consisting of heart, blood vessels, and blood cells is the first functional organ to develop. This lecture will focus on the formation of the embryonic vasculature, the origin and formation of the early heart tube and primitive cardiac chambers, cardiac looping, and the primitive circulation. Between the 5th - 8th week of embryonic development, the tubular heart is remodeled into a four chambered structure. We will see how right and left atrioventricular canals connect each atrium with its respective ventricle, and how the atrial septum and definitive right and left atria form. We will also see why the great veins deliver blood to the right atrium while the pulmonary veins empty into the left. GLOSSARY: Angioblasts: precursors of blood vessels Angiogenesis: lengthening, branching, sprouting and remodeling of embryonic blood vessels Aortic arches: paired arteries surrounding the pharynx; portions will contribute to formation of the great arterial vessels Blood islands: clusters of cells in the yolk sac, connecting stalk and chorionic villi that form primitive blood vessels Cardiac jelly: gelatinous extracellular matrix that forms the middle layer of the heart tube Ductus venosus: shunts most of the blood in the umbilical vein into the inferior vena cava -

Cardiovascular System - Accessscience from Mcgraw-Hill Education
Cardiovascular system - AccessScience from McGraw-Hill Education http://accessscience.com/content/109900 (http://accessscience.com/) Article by: Weichert, Charles K. College of Arts and Sciences, University of Cincinnati, Cincinnati, Ohio. Copenhaver, W. M. College of Physicians and Surgeons, Columbia University, New York; Department of Biological Structures, School of Medicine, University of Miami, Miami, Florida. Ebert, James D. Department of Embryology, Carnegie Institution, Washington, DC. Patten, Bradley M. Department of Anatomy, University of Michigan, Ann Arbor, Michigan. Jones, David R. Department of Zoology, University of British Columbia, Vancouver, Canada. Publication year: 2014 DOI: http://dx.doi.org/10.1036/1097-8542.109900 (http://dx.doi.org/10.1036/1097-8542.109900) Content Comparative Anatomy Embryogenesis of blood vessels Balancing ventricular output Heart Angiogenesis Human Postnatal Circulation Arterial system Circulatory system morphogenesis Pulmonary circuit and ductus Venous system Primitive venous system Physiological aspects of transition Comparative Embryology Functional Development of Heart Comparative Physiology Heart Contractions of the heart General physiology of circulation Tubular heart formation Heart-forming areas Microcirculation Cardiac loop and regional development Contractile proteins Heart Formation of definitive heart Synthesis of contractile proteins Arteries Partitioning of mammalian heart Action of inhibitors Venous system Division of atrium and ventricles Human Fetal Circulation at Term Bibliography