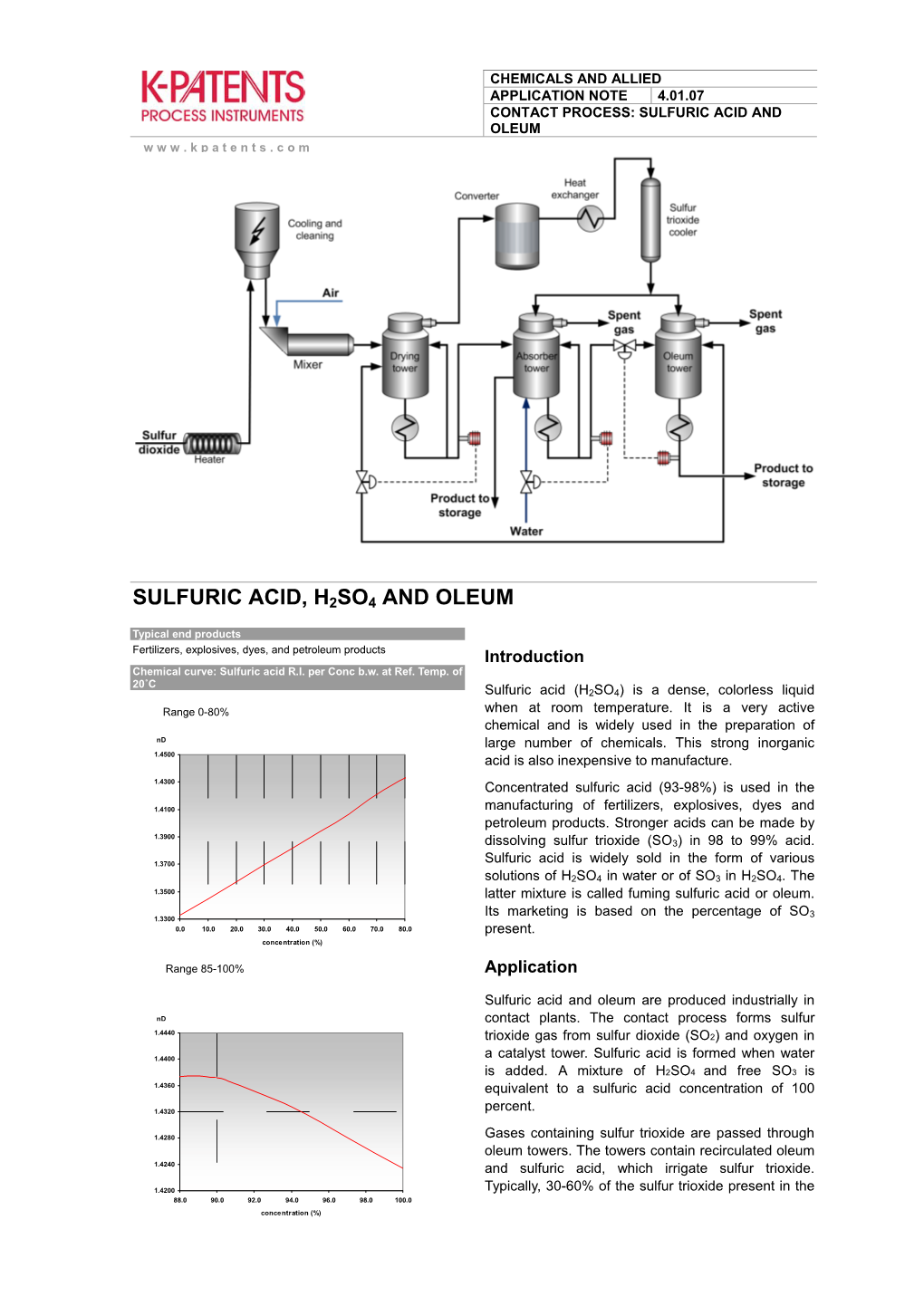
Sulfuric Acid, H2so4 and Oleum
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sulfuric Acid from Sulfur Updates on Contact Process PEP Review 2018-12 February 2018
` Sulfuric Acid from Sulfur Updates on Contact Process PEP Review 2018-12 February 2018 Rajiv Narang Director Process Economics Program IHS Markit | PEP Review 2018-12 Sulfuric Acid from Sulfur/Updates on Contact Process PEP Review 2018-12 Sulfuric Acid from Sulfur/Updates on Contact Process Rajiv Narang, Director Abstract Sulfuric acid (H2SO4) is the world’s largest consumed chemical, with consumption of around 260 MMT (million metric tons) on a 100% acid basis and a growth rate of around 2%. Traditionally, consumption of this chemical is considered as a barometer of a nation’s GDP. The majority of sulfuric acid production (59%) is from burning of elemental sulfur, in a contact process. The sulfur is sourced mostly from oil and gas processing facilities, in which the sulfur is removed from various petroleum or natural gas products. IHS Markit’s Process Economics Program (PEP) last addressed this production technology in PEP Report 84A, Sulfuric Acid (June 1985), which covered the manufacture of sulfuric acid from sulfur in new versus old, retrofitted plants, as well as from metallurgical offgases. This review specifically updates the contact process for the production of sulfuric acid by burning of elemental sulfur. The review examines the developments in this production technology, including advances in catalyst, material of construction, and heat recovery. The process is simulated using Aspen Plus™ software. It focuses on technology basis, raw material and utility consumptions, equipment list, capital cost, along with capacity exponents, and production costs for a 2,000 STPD (short ton per day) of (100% basis) sulfuric acid product. This review provides insight into sulfuric acid plant process economics, and can be used as a tool for cost estimation for different plant capacities. -

Catalyst Precursors for Hydrodesulfurization Synthesized in Supercritical Fluids Manuel Théodet
New generation of ”bulk” catalyst precursors for hydrodesulfurization synthesized in supercritical fluids Manuel Théodet To cite this version: Manuel Théodet. New generation of ”bulk” catalyst precursors for hydrodesulfurization synthesized in supercritical fluids. Material chemistry. Université Sciences et Technologies - Bordeaux I,2010. English. NNT : 2010BOR14092. tel-00559113 HAL Id: tel-00559113 https://tel.archives-ouvertes.fr/tel-00559113 Submitted on 24 Jan 2011 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution - NonCommercial - NoDerivatives| 4.0 International License N° d’ordre : 4092 THÈSE présentée à L’UNIVERSITÉ BORDEAUX I ÉCOLE DOCTORALE DES SCIENCES CHIMIQUES Par Manuel THEODET Ingénieur ENSCPB POUR OBTENIR LE GRADE DE DOCTEUR SPÉCIALITÉ : Physico-Chimie de la Matière Condensée ___________________ NOUVELLE GENERATION DE PRECURSEURS « BULK » DE CATALYSEUR D’HYDRODESULFURATION SYNTHETISES EN MILIEU FLUIDE SUPERCRITIQUE ___________________ NEW GENERATION OF « BULK » CATALYST PRECURSORS FOR HYDRODESULFURIZATION SYNTHESIZED IN SUPERCRITICAL FLUIDS ___________________ Co-superviseurs de recherche : Cristina Martínez & Cyril Aymonier Soutenue le 03 Novembre 2010 Après avis favorable de : M. E. PALOMARES, Professor, UPV, Valencia, Spain Rapporteurs M. M. TÜRK, Professor, KIT, Karlsruhe, Germany Devant la commission d’examen formée de : M. -

PROCESS MODELING and ANALYSIS of CO2 PURIFICATION for OXY-COAL COMBUSTION MASSACHUSETTS INS E by of TECHNOLOGY
PROCESS MODELING AND ANALYSIS OF CO2 PURIFICATION FOR OXY-COAL COMBUSTION MASSACHUSETTS INS E By OF TECHNOLOGY Chukwunwike Ogbonnia Iloeje MAY 18 2011 B.Eng. Mechanical Engineering LIBRRIES University of Nigeria, Nsukka, 2004 SUBMITTED TO THE DEPARTMENT OF MECHANICAL ENGINEERING IN AR(MES PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE IN MECHANICAL ENGINEERING AT THE MASSACHUSETTS INSTITUTE OF TECHNOLOGY JANUARY 2011 0 2011 Massachusetts Institute of Technology. All rights reserved. The author hereby grants to MIT permission to reproduce and to distribute publicly paper and electronic copies of this thesis document in whole or in part in any medium now known or hereafter created. Signature of Author........... ............................... Department of Mechanical Engineering January 14, 2011 Certified by.............. Ahmed F. Ghoniem Ronald C. Crane Professor Thacic Cunanwicnr Accepted by.................................. .... ................. Dave E.Hardt Chairman, Department Committee on Graduate Students *r PROCESS MODELING AND ANALYSIS OF CO2 PURIFICATION FOR OXY-COAL COMBUSTION By Chukwunwike Ogbonnia Iloeje Submitted to the Department of Mechanical Engineering on January 14, 2011 in Partial Fulfillment of the Requirements for the degree of Master of Science in Mechanical Engineering ABSTRACT Oxy-coal combustion technology has great potential as one of the major CO2 capture technologies for power generation from coal. The distinguishing feature of oxy-coal combustion is that the oxygen source is a high concentration oxygen stream and the product flue gas consists primarily of CO2 and H20 with contaminants like NOx, SOx, and non-condensable gases like argon, oxygen and nitrogen. For carbon sequestration and Enhanced Oil Recovery (EOR) applications, pipeline transport standards as well as storage specifications impose concentration limits on these contaminants. -

Chemical Reactions
INTRODUCTION:- Sulfuric acid (alternative spelling sulphuric acid) is a highly corrosive strong mineral acid with the molecular formula H2SO4. It is a pungent-ethereal, colorless to slightly yellow viscous liquid which is soluble in water at all concentrations.Sometimes, it is dyed dark brown during production to alert people to its hazards.The historical name of this acid is oil of vitriol. Pure sulfuric acid is not encountered naturally on Earth in anhydrous form, due to its great affinity for water. Dilute sulfuric acid is a constituent of acid rain, which is formed by atmospheric oxidation of sulfur dioxide in the presence of water – i.e., oxidation of sulfurous acid. Sulfur dioxide is the main byproduct produced when sulfur-containing fuels such as coal or oil are burned. Sulfuric acid is formed naturally by the oxidation of sulfide minerals, such as iron sulfide. The resulting water can be highly acidic and is called acid mine drainage (AMD) or acid rock drainage (ARD). This acidic water is capable of dissolving metals present in sulfide ores, which results in brightly colored, toxic streams. Alternative Names:- 1. Oil of vitriol 2. Sulfuric acid 3. Vitriol 4. Battery acid 5. Electrolyte acid PHYSICAL PROPERTIES:- Appearance colourless, oily, corrosive liquid Melting point 10 deg C Molecular Formula H2O4S 337 deg C Boiling point 1mm of Hg at 145.8 deg C Vapor pressure 1.840 g/mL Density <0.3 (25 °C, vs air) vapor density 1.01 pH of 10 % of solution Non flammable Flammability limits 98.07848 g/mol Molar weight CHEMICAL PROPERTIES :- Attacks most metals. -

Modelling and Multi-Objective Optimization of the Sulphur Dioxide Oxidation to the Sulphur Trioxide Process
Modelling and multi-objective optimization of the sulphur dioxide oxidation to the sulphur trioxide process by Mohammad Reza Zaker Thesis submitted to the University of Ottawa in partial Fulfillment of the requirements for the Master of Applied Science Department of Chemical and Biological Engineering Faculty of Engineering University of Ottawa © Mohammad Reza Zaker, Ottawa, Canada, 2020 Abstract Abstract In this thesis, the catalytic oxidation of sulphur dioxide (SO2) to sulphur trioxide (SO3), which is a critical step in the production of sulphuric acid (H2SO4), was studied under adiabatic operating conditions. The oxidation process is taking place in a heterogeneous plug flow reactor. Because the SO2 oxidation is a highly exothermic equilibrium reaction, a series of packed bed catalytic reactors with intercooling heat exchangers is required to achieve high SO2 conversion. To predict the effect of the operating conditions such as the temperature and the pressure on the oxidation as well as to model mathematically the reactor, it is essential to find an appropriate kinetic rate equation. In this study, various kinetic models were evaluated to select the kinetic model that appeared to be the most representative of available experimental data. In this regard, the residual sum of squares of the differences between the predicted and experimental conversion values was used to compare the various kinetic models. The model which showed the better fitting of the experimental data was the one proposed by Collina et al. The SO2 oxidation reactor model was developed in order to propose a methodology to perform the multi-objective optimization of many process strategies involving a number of catalytic beds and different reactor configurations. -

Unisim-Design Simulation and Analysis of a Sulphuric Acid Manufacturing Plant with Double Absorption Process
UniSim-Design Simulation and Analysis of a Sulphuric Acid Manufacturing Plant with Double Absorption Process Amine Mounaam1,2, Yasser Harmen2,4, Younes Chhiti2,3, Ahmed Souissi1,2, Mohamed Salouhi1 and Mohamed El Khouakhi2 1All Laboratory, Ecole Mohammadia d’Ingénieurs, Mohammed V University, Rabat, Morocco 2Innovation Lab for Operations, Mohammed VI Polytechnic University, Ben Guérir, Morocco 3Ibn Tofail University, Kenitra, Morocco 4Science Engineer Laboratory for Energy (LabSIPE), National School of Applied Sciences, Chouaib Doukkali University, El Jadida, Morocco [email protected], [email protected] Keywords: Sulphuric Acid, Modelling, Simulation, UniSim-Design, Chemical Reactions, Double Absorption Process, UniSim-Thermo, Peng-Robinson, NRTL. Abstract: In the sulphuric acid manufacturing industries, plant modelling and simulation is a challenging task to minimize emissions, maximize production performance and revenue. In this context, this study presents the steady behaviour of a double absorption process of an industrial sulphuric acid plant. The closed-loop process is modelled and simulated using UniSim Design R451 simulator and validated with plant data. The model includes principally: conversion reactor, plug flow reactors, absorbers, heat exchangers, pumps and compressors. The parameters of the converter kinetic were fitted to the real plant data, while the other parameters were estimated using conventional correlations. The results show a good agreement for the complete plant, with an accuracy that exceeds 97 %. Besides the optimization aspects, UniSim Design plant model is also useful for operator training, simulation of diverse scenarios and development of processes digital twin. 1 INTRODUCTION process was introduced to achieve 99.5 % or higher conversion rate, whereas the unreacted SO2 and SO3 Worldwide, sulphuric acid is the most used chemical are released to the environment (Moeller & Winkler, products in the basic chemical industry (Moats et al., 1968). -

Oleum, Sulfur Trioxide, and Sulfuric Acid
Oleum, Sulfur Trioxide, and Sulfuric Acid These three very corrosive chemicals are closely related. Oleum is cloudy, oily, fuming liquid or sometimes a solid which releases sulfur trioxide in contact with air as in a spill. This sulfur trioxide reacts quickly with any air moisture producing a fine sulfuric acid mist. Inhalation at low concentrations for a short period irritates the nose, throat, and lungs. Prolonged exposure or higher concentrations causes a burning sensation, coughing, gagging, chest pain, fluid in lungs, and possible suffocation and death. The effects of inhalation may be delayed. The mist also severely irritates eyes and skin. We will look at these chemicals, its uses, and examine several accidents involving oleum spills. Oleum spills are very dangerous because chemical contact can “suck” the water out of organic materials leaving a black char generating a lot of heat and possibly resulting in fire. If water is sprayed on the chemical, a sulfuric acid mist will likely be formed which is difficult to control and dangerous to inhale. Physical Properties of Oleum, Sulfur Trioxide, and Sulfuric Acid Oleum is excess sulfur trioxide dissolved in sulfuric acid. Another name for Oleum is “Sulfuric acid, fuming”. It is sometimes shipped by railcar under UN 1831. The chemical may also be transported tanker truck, pipeline or in smaller containers. The Emergency Response Guidebook under UN 1831 makes a distinction between “Sulfuric acid, fuming, with less than 30% free Sulfur trioxide” and “Sulfuric acid, with not less than 30% free Sulfur trioxide”. For example, a tank car containing 90 tons of oleum with 30% free sulfur trioxide contains 60 tons of sulfuric acid and 30 tons of free sulfur trioxide dissolved in the sulfuric acid. -

Introduction to Wet Sulfuric Acid Plants Optimization Through Exergoeconomics
Introduction to Wet Sulfuric Acid Plants Optimization Through Exergoeconomics Master’s thesis Borja Xicoy Almirall Professor: Prof. Dr.-Ing. Günter Wozny Tutor: Dipl.-Ing. Jan Schöneberger June 2009 Kurzfassung Die Diplomarbeit ist eine Einleitung zur thermoökonomische Analyse in Schwe- felsäure Anlagen. Eine Referenzanlage mit der folgenden charakteristiken wird analysiert: eine Schwefelbrennkammer zur Oxidation schwefelwasserstoffhaltiger Gase, ein durch Luftzufuhr abgekühlter Naß-Katalyse Prozess und eine Absorptionskolonne zur Produzierung von Schwefelsäure mit 78 Massenprozent H2SO4. Außerdem wird Wasserdampf von 40 bar und 5 bar erzeugt. Die thermoökonomische Analyse besteht aus drei Schritten: Zuerst wird eine Ex- ergieanalyse unter Anwendung der kommerziellen Software CHEMCAD und des Hil- fsprogramms CHEMEX durchgeführt. CHEMEX wurde im Rahmen dieser Diplo- marbeit entwickelt und dient zur Berechnung der chemischen Exergie von Elek- trolytlösungen. Im nächsten Schritt wird eine Wirtschaftlichkeitsanalyse zur der Berechnung der nivellierten Kosten der Anlage mittels PEC und weiteren Kosten- schätzungsmethoden durchgeführt. Im dritten Schritt der Analyse werden Exergie- und Wirtschaftlichkeitsanalyse durch die Aufteilung der nivellierten Kosten auf alle Strömen der gesamten Anlage bezüglich ihrer Exergiewerte kombiniert. Dafür ist ein lineares Gleichungssystem mit den Kostbilanzen jeweiliger Analgenkomponente und komponentenspezifischen Hilfsgleichungen zu lösen. Schließlich werden drei kosteneffektive Optimierungsschritte auf die Referenzanlage angewendet, wobei ex- ergoökonomische Kennzahlen die jeweiligen Optimierungsschritte motivieren. i Abstract This thesis pretends to be an introduction to the use of thermoeconomics in sulfuric acid plants. A reference plant with the followings features is examined: a sulfur burner which oxidizes hydrogen sulfide gases, a wet-catalysis process which is cooled by means of air quenching, and an absorbtion column that produces sulfuric acid of 78 wt.%. -

Advanced Process Analysis System
ADVANCED PROCESS ANALYSIS SYSTEM VOL. I A Thesis Submitted to the Graduate Faculty of the Louisiana State University and Agriculture and Mechanical College in partial fulfillment of the requirements for the degree of Master of Science in Chemical Engineering in The Department of Chemical Engineering by Kedar Shivanand Telang B.S., Indian Institute of Technology, Bombay, 1996 December 1998 ACKNOWLEDGMENTS The author wishes to express his appreciation and gratitude to Professor Ralph W. Pike for giving him the opportunity to participate in this research work. His guidance and assistance are deeply appreciated. The examining committee members, Professor F. Carl Knopf and Professor Karsten Thompson are recognized for their efforts in reviewing and evaluating this research. The cooperation of engineers and managers of the IMC Agrico Company was invaluable in this research. The assistance of Mr. Thomas A. Hertwig of IMC Agrico is especially acknowledged for providing the detailed information and data for the sulfuric acid process. In addition, the assistance of Ms. Gayathri Srinivasan and Mr. Tai Lee in Visual Basic programming was invaluable. The financial support for this research by the Environment Protection Agency is gratefully acknowledged. Also, the Department of Chemical Engineering at Louisiana State University is recognized for its assistance and support. Finally, the author would like to acknowledge the support and encouragement of friends and family during his post graduate study. ii TABLE OF CONTENTS VOLUME I ACKNOWLEDGEMENTS..........................................................................................ii -
The Contact Process
CONTACT PROCESS Sulfuric acid is one of the most important industrial chemicals Outline three uses of sulfuric acid in industry 1. The major use of sulfuric acid in Australia is in the manufacture of fertilizers such as ammonium sulfate and superphosphate. Superphosphate is produced by reacting sulfuric acid with rock phosphate. Ammonium sulfate is produced by neutralising ammonia with sulfuric acid. 2. Production of titanium (IV) oxide from titanium minerals eg ilmenite. Titanium is an important lightweight metal used to produce strong alloys and white, opaque pigments. H2SO4 is used to leach the titanium from the minerals after mining. 3. Cleaning iron – because very corrosive used to remove the oxide layer from iron or steel before they are galvanised or electroplated. Describe the processes used to extract sulfur from mineral deposits, identifying the properties of sulfur which allow its extraction and analyzing potential environmental issues that may be associated with its extraction - Most sulphur is extracted from mineral deposits using the Frasch process. Superheated stream is pumped down the outer of 3 concentric pipes into the sulphur deposit, and since sulphur has a low melting point (119) it is readily melted. At the same time, compressed air is blown down the inner pipe, and because sulphur has a relatively low density, the air is able to force the molten sulphur up the middle pipe to the surface where it resolidifies. The insolubility of sulphur in water means that it separates from any water, leaving 99.5% pure sulphur. - Sulfur is also obtained from hydrogen sulphide in natural gas and petroleum. -

Petroleum Refining: Introduction, Products, Refinery's Configurations, Hydrogen Technologies
Faculty of Chemical Engineering and Technology University of Zagreb Petroleum Refining: Introduction, Products, Refinery's Configurations, Hydrogen Technologies Ante Jukić ZAVOD ZA TEHNOLOGIJU NAFTE I PETROKEMIJU HR-10000 Zagreb, Savska cesta 16, p.p. 177 / Tel. +385-1-4597125 / E-adresa: [email protected] Gasoline, Diesel, and LPG Technologies In this section we will be describing the different refining technologies relating to Gasoline, Diesel and LPG. Although these are labeled as conventional technologies, refineries today have become increasingly more efficient and efforts across the globe are being made to invest in better performing technologies and processes that save energy and decrease each refineries environmental impact whilst meeting increasingly more stringent fuel product specifications. As can be seen below a basic refinery typically produces a variety of products including: LPG; petrochemicals; gasoline; jet fuel; paraffin for lighting and heating; lubricating oils, waxes and polishes; heavy fuel oil, and bitumen for roads and roofing. Products Produced by Refineries Processing units used in refineries • Crude Oil Distillation unit: distills the incoming crude oil into various fractions for further processing. • Vacuum Distillation unit: further distills the residue oil from the bottom of the crude oil distillation unit. • Naphtha Hydrotreater unit: uses hydrogen to desulfurize the naphtha fraction from the crude oil distillation or other units within the refinery. • Catalytic Reforming unit: converts the desulfurized naphtha molecules into higher-octane molecules to produce reformate, which is a component of the end-product gasoline or petrol. • Alkylation unit: converts isobutane and butylenes into alkylate, which is a very high-octane component of the end-product gasoline or petrol. -

8.10 Sulfuric Acid 8.10.1 General
8.10 Sulfuric Acid 8.10.1 General1-2 Sulfuric acid (H2SO4) is a basic raw material used in a wide range of industrial processes and manufacturing operations. Almost 70 percent of sulfuric acid manufactured is used in the production of phosphate fertilizers. Other uses include copper leaching, inorganic pigment production, petroleum refining, paper production, and industrial organic chemical production. Sulfuric acid may be manufactured commercially by either the lead chamber process or the contact process. Because of economics, all of the sulfuric acid produced in the U. S. is now produced by the contact process. U. S. facilities produce approximately 42 million megagrams (Mg) (46.2 million tons) of H2SO4 annually. Growth in demand was about 1 percent per year from 1981 to 1991 and is projected to continue to increase at about 0.5 percent per year. 8.10.2 Process Description3-5 Since the contact process is the only process currently used, it will be the only one discussed in this section. Contact plants are classified according to the raw materials charged to them: elemental sulfur burning, spent sulfuric acid and hydrogen sulfide burning, and metal sulfide ores and smelter gas burning. The contributions from these plants to the total acid production are 81, 8, and 11 percent, respectively. The contact process incorporates 3 basic operations, each of which corresponds to a distinct chemical reaction. First, the sulfur in the feedstock is oxidized (burned) to sulfur dioxide (SO2): → S O2 SO2 (1) The resulting sulfur dioxide is fed to a process unit called a converter, where it is catalytically oxidized to sulfur trioxide (SO3): → 2SO2 O2 2SO3 (2) Finally, the sulfur trioxide is absorbed in a strong 98 percent sulfuric acid solution: → SO3 H2O H2SO4 (3) 8.10.2.1 Elemental Sulfur Burning Plants - Figure 8.10-1 is a schematic diagram of a dual absorption contact process sulfuric acid plant that burns elemental sulfur.