Surgical Management of Intracranial Capillary Hemangiomas in Children: Report of 2 Cases
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Kaposiform Hemangioendothelioma with Kasabach-Merritt Syndrome Mistaken for Child Abuse in a Newborn
Kaposiform Hemangioendothelioma With Kasabach-Merritt Syndrome Mistaken for Child Abuse in a Newborn Amanda A. Cyrulnik, MD; Manju C. Dawkins, MD; Gert J. Smalberger, MD; Scott Young, MD; Ranon E. Mann, MD; Mark I. Jacobson, MD; Adam J. Friedman, MD Practice Points Vascular tumors in dermatology may mimic child abuse. Even when all signs favor abuse, a nonresolving lesion should alarm clinicians to consider an alter- nate diagnosis. Kasabach-Merritt syndrome is a serious and potentially life-threatening condition that requires prompt evaluation. Kaposiform hemangioendotheliomaCUTIS is a rare vas- elevated D-dimer levels, confirming a diagnosis of cular neoplasm of childhood that may have an Kasabach-Merritt syndrome (KMS). alarming and potentially misleading clinical pre- Cutis. 2014;93:E17-E20. sentation. Awareness of this entity is important to provide appropriate and immediate medical care. Case Report We report the case of a 24-day-old female new- A 24-day-old female newborn presented to a hospi- born who presented with a large bruiselike lesion tal with a large bruiselike lesion on the left leg. A on theDo left leg. A diagnosis ofNot cellulitis suspected diagnosis Copy of cellulitis suspected to be secondary to to be secondary to child abuse was made and the child abuse was made and the patient subsequently patient subsequently was placed in foster care; was placed in foster care; however, the lesion did not however, the lesion did not resolve after treatment resolve after treatment and relocation. At 69 days of and relocation. On reevaluation at our institution, age, the patient was readmitted, now to our hospi- physical examination revealed a round, 34-cm, tal, after the lesion persisted and had progressively violaceous, indurated, fixed, nonblanching, non- expanded. -

Benign Hemangiomas
TUMORS OF BLOOD VESSELS CHARLES F. GESCHICKTER, M.D. (From tke Surgical Palkological Laboratory, Department of Surgery, Johns Hopkins Hospital and University) AND LOUISA E. KEASBEY, M.D. (Lancaster Gcaeral Hospital, Lancuster, Pennsylvania) Tumors of the blood vessels are perhaps as common as any form of neoplasm occurring in the human body. The greatest number of these lesions are benign angiomas of the body surfaces, small elevated red areas which remain without symptoms throughout life and are not subjected to treatment. Larger tumors of this type which undergb active growth after birth or which are situated about the face or oral cavity, where they constitute cosmetic defects, are more often the object of surgical removal. The majority of the vascular tumors clinically or pathologically studied fall into this latter group. Benign angiomas of similar pathologic nature occur in all of the internal viscera but are most common in the liver, where they are disclosed usually at autopsy. Angiomas of the bone, muscle, and the central nervous system are of less common occurrence, but, because of the symptoms produced, a higher percentage are available for study. Malignant lesions of the blood vessels are far more rare than was formerly supposed. An occasional angioma may metastasize following trauma or after repeated recurrences, but less than 1per cent of benign angiomas subjected to treatment fall into this group. I Primarily ma- lignant tumors of the vascular system-angiosarcomas-are equally rare. The pathological criteria for these growths have never been ade- quately established, and there is no general agreement as to this par- ticular form of tumor. -

8 Liver Hemangioma
Liver Hemangioma 101 8 Liver Hemangioma Valérie Vilgrain and Giuseppe Brancatelli CONTENTS 8.1 Introduction 8.1 Introduction 101 8.2 Sonography 101 8.3 Computed Tomography 102 Hemangioma is the most common benign hepatic 8.4 Magnetic Resonance 104 tumor. The prevalence of hemangioma in the general 8.5 Scintigraphy 107 population ranges from 1%–2% to 20% (Semelka and 8.6 Percutaneous Biopsy 107 Sofka 1997). The female-to-male ratio varies from 8.7 Atypical Patterns 108 2:1 to 5:1. They occur at all ages. The vast majority of 8.7.1 Hemangioma with Echoic Border 108 8.7.2 Large, Heterogeneous Hemangioma 108 hemangiomas remain clinically silent. Few patients 8.7.3 Rapidly Filling Hemangioma 108 are symptomatic due to a mass lesion, complications 8.7.4 Very Slow Filling Hemangioma 110 or compression of adjacent structures. Most of these 8.7.5 Calcifi ed Hemangioma 110 symptoms are observed in large hemangiomas. The 8.7.6 Hyalinized Hemangioma 110 natural history of hemangiomas is variable: most of 8.7.7 Cystic or Multilocular Hemangioma 111 8.7.8 Hemangioma with Fluid–Fluid Level 111 them remain stable, some may grow or involute. The 8.7.9 Pedunculated Hemangioma 111 role of sex hormones in causing enlargement during 8.7.10 Hemangioma with pregnancy or recurrence is disputed. Arterial-Portal Venous Shunt 111 Hemangiomas are usually solitary, less than 5 cm 8.7.11 Hemangioma with Capsular Retraction 111 in size and appear as well-delineated lesions of red 8.8 Hemangioma Developing in Abnormal Liver 112 color that partially collapse on sectioning. -
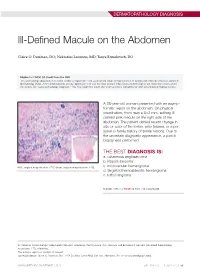
Ill-Defined Macule on the Abdomen
DERMATOPATHOLOGY DIAGNOSIS Ill-Defined Macule on the Abdomen Claire O. Dorfman, DO; Nektarios Lountzis, MD; Tanya Ermolovich, DO Eligible for 1 MOC SA Credit From the ABD This Dermatology Diagnosis in our print edition is eligible for 1 self-assessment credit for Maintenance of Certification from the American Board of Dermatology (ABD). After completing this activity, diplomates can visit the ABD website (http://www.abderm.org) to self-report the credits under the activity title “Cutis Dermatology Diagnosis.” You may report the credit after each activity is completed or after accumulating multiple credits. A 38-year-old woman presented with an asymp- tomatic lesion on the abdomen. On physical examination, there was a 5×2-mm, solitary, ill- defined pink macule on the right side of the abdomen. The patient denied recent change in size or color of the lesion, prior trauma, or a per- sonal or family history of similar lesions. Due to the uncertain diagnostic appearance, a punch biopsy was performed. THE BEST DIAGNOSIS IS: a. cutaneous angiosarcoma b. Kaposi sarcoma H&E, original magnification ×200 (inset, original magnification ×40). c. microvenular hemangioma d. targetoid hemosiderotic hemangioma e. tufted angioma PLEASE TURN TO PAGE 68 FOR THE DIAGNOSIS Dr. Dorfman is from Lehigh Valley Health Network, Allentown, Pennsylvania. Drs. Lountzis and Ermolovich are from Advanced Dermatology Associates, LTD, Allentown. The authors report no conflict of interest. Correspondence: Claire O. Dorfman, DO, 1259 S Cedar Crest Blvd, Ste 100, Allentown, PA 18103 ([email protected]). WWW.MDEDGE.COM/DERMATOLOGY VOL. 104 NO. 1 I JULY 2019 53 DERMATOPATHOLOGY DIAGNOSIS DISCUSSION THE DIAGNOSIS: Microvenular Hemangioma icrovenular hemangioma is an acquired benign vas- from pink patches to dark purple plaques or nodules that cular neoplasm that was described by Hunt et al1 in commonly occur on the lower legs10; however, the clinical M1991, though Bantel et al2 reported a similar entity appearance of KS varies depending on the clinical variant termed micropapillary angioma in 1989. -

Vascular Lesions to Look out For
10/25/19 Vascular Lesions to Look Out For Adnan Mir, MD, PhD Dermpath Diagnostics Port Chester, New York Assistant Professor Pediatric Dermatology and Dermatopathology Dermpath New York Medical College Diagnostics Disclosures I have no relevant financial conflicts of interest I will not mention off-label uses of FDA-approved medications 62 year old man Site: Left cheek Clinical: Facial fullness for seven months Asympatomatic R/o Morbihan, rosacea, seborrheic dermatitis 1 10/25/19 61 year old woman Site: Left cheek Clinical: Bleeding ulcer on abdomen Obesity, Pulmonary HTN Harker et al, J Cut Pathol 2017 Jan;44(1) 2 10/25/19 Cutaneous Angiosarcoma Angiosarcoma of the face/scalp first described in 1964 as malignant angioendothelioma of the skin 1% of sarcomas, 1/100,000 incidence Distinct behavior and presentation among angiosarcomas: Face, scalp – Wilson-Jones Lymphedema – Stewart-Treves Radiation-induced Epithelioid 3 10/25/19 Cutaneous Angiosarcoma Clinical presentation Typically asymptomatic Bruise-like Violaceous papules, plaques, nodules Clinical impressions often: Hematoma, rosacea, eczema, seborrheic dermatitis, hemangioma, xanthelasma, cellulitis, angioedema Cutaneous Angiosarcoma Epidemiology and predisposing factors Average age 70 M:F = 2:1 Chronic sun exposure? Overall, unknown etiology Cutaneous Angiosarcoma Pathophysiology Mutations detected in P53 (>50%) KRAS PTPRB Angiogenesis PLCG1 Upregulated: NTSR1, ANKRD1, CDKN2C, KDR 4 10/25/19 Cutaneous Angiosarcoma Histologic features Infiltrative growth pattern Well-differentiated -

Diagnosis and Management of Infantile Hemangioma D
CORE Metadata, citation and similar papers at core.ac.uk Provided by Hofstra Northwell Academic Works (Hofstra Northwell School of Medicine) Donald and Barbara Zucker School of Medicine Journal Articles Academic Works 2015 Diagnosis and Management of Infantile Hemangioma D. H. Darrow A. K. Greene A. J. Mancini A. J. Nopper T. M. O Northwell Health Follow this and additional works at: https://academicworks.medicine.hofstra.edu/articles Part of the Otolaryngology Commons Recommended Citation Darrow D, Greene A, Mancini A, Nopper A, O T. Diagnosis and Management of Infantile Hemangioma. 2015 Jan 01; 136(4):Article 575 [ p.]. Available from: https://academicworks.medicine.hofstra.edu/articles/575. Free full text article. This Article is brought to you for free and open access by Donald and Barbara Zucker School of Medicine Academic Works. It has been accepted for inclusion in Journal Articles by an authorized administrator of Donald and Barbara Zucker School of Medicine Academic Works. CLINICAL REPORT Guidance for the Clinician in Rendering Pediatric Care Diagnosis and Management of Infantile Hemangioma: Executive Summary David H. Darrow, MD, DDS, Arin K. Greene, MD, Anthony J. Mancini, MD, Amy J. Nopper, MD, the SECTION ON DERMATOLOGY, SECTION ON OTOLARYNGOLOGY–HEAD & NECK SURGERY, AND SECTION ON PLASTIC SURGERY INTRODUCTION Infantile hemangiomas (IHs) are the most common tumors of childhood. Unlike other tumors, they have the capacity to involute after proliferation, often leading primary care providers to assume they will resolve without intervention or consequence. However, a subset of IHs may be associated with complications, resulting in pain, functional impairment, or permanent disfigurement. -

Diagnosis of Bacillary Angiomatosis and Kaposi´S Sarcoma in the Same Skin Lesion in a Patient with Recent HIV/AIDS Diagnosis
Clinical Dermatology: Research and Therapy Open Access Case Report Diagnosis of Bacillary Angiomatosis and Kaposi´s Sarcoma in the same Skin Lesion in a Patient with Recent HIV/AIDS Diagnosis Correa J1,2*, Villafane MF1,2, De Carolis L1,2, Corti M1,2, Sforza R1, Maronna E3, Giamperetti S4 and Bare P5 1HIV/AIDS Division, Infectious Diseases F. J. Muniz Hospital, Argentina 2Department of Medicine, University of Buenos Aires, Argentina 3Histopathology Laboratory, Infectious Diseases F. J. Muniz Hospital, Argentina 4Zoopathology Laboratory, Infectious Diseases F. J. Muniz Hospital, Argentina 5Virology Laboratory, National Academy of Medicine, Argentina A R T I C L E I N F O A B S T R A C T Article history: Received: 21 September 2017 Kaposi´s Sarcoma (KS) is polyclonal multifocal disease of endothelial origin, Accepted: 27 November 2017 Published: 05 December 2017 with four clinical and epidemiological well described variants. AIDS-related Keywords: KS has a variable clinical course, ranging from minimal disease located to the Bacillary angiomatosis; Kaposi´s sarcoma; skin and presenting as an incidental finding to a rapidly progressing neoplasm HIV/AIDS with skin, mucosal, and visceral compromise that can result in significant Copyright: © 2017 Correa J et al., morbidity and mortality, depending upon the specific sites of involvement. Clin Dermatol Res Ther This is an open access article distributed Bacillary Angiomatosis (BA) is a rare manifestation of infection caused by under the Creative Commons Attribution License, which permits unrestricted use, Bartonella species, which leads to vasoproliferative lesions of skin and other distribution, and reproduction in any medium, provided the original work is organs. -

Vascular Malformations and Tumors Continues to Grow Overview Table Vascular Anomalies
ISSVA classification for vascular anomalies © (Approved at the 20th ISSVA Workshop, Melbourne, April 2014, last revision May 2018) This classification is intended to evolve as our understanding of the biology and genetics of vascular malformations and tumors continues to grow Overview table Vascular anomalies Vascular tumors Vascular malformations of major named associated with Simple Combined ° vessels other anomalies Benign Capillary malformations CVM, CLM See details See list Lymphatic malformations LVM, CLVM Locally aggressive or borderline Venous malformations CAVM* Arteriovenous malformations* CLAVM* Malignant Arteriovenous fistula* others °defined as two or more vascular malformations found in one lesion * high-flow lesions A list of causal genes and related vascular anomalies is available in Appendix 2 The tumor or malformation nature or precise classification of some lesions is still unclear. These lesions appear in a separate provisional list. For more details, click1 on Abbreviations used the underlined links Back to ISSVA classification of vascular tumors 1a Type Alt overview for previous view Benign vascular tumors 1 Infantile hemangioma / Hemangioma of infancy see details Congenital hemangioma GNAQ / GNA11 Rapidly involuting (RICH) * Non-involuting (NICH) Partially involuting (PICH) Tufted angioma * ° GNA14 Spindle-cell hemangioma IDH1 / IDH2 Epithelioid hemangioma FOS Pyogenic granuloma (also known as lobular capillary hemangioma) BRAF / RAS / GNA14 Others see details * some lesions may be associated with thrombocytopenia -

Diagnosis and Management of Infantile Hemangioma David H
CLINICAL REPORT Guidance for the Clinician in Rendering Pediatric Care Diagnosis and Management of Infantile Hemangioma David H. Darrow, MD, DDS, Arin K. Greene, MD, Anthony J. Mancini, MD, Amy J. Nopper, MD, the SECTION ON DERMATOLOGY, SECTION ON OTOLARYNGOLOGY–HEAD AND NECK SURGERY, and SECTION ON PLASTIC SURGERY abstract Infantile hemangiomas (IHs) are the most common tumors of childhood. Unlike other tumors, they have the unique ability to involute after proliferation, often leading primary care providers to assume they will resolve without intervention or consequence. Unfortunately, a subset of IHs rapidly develop complications, resulting in pain, functional impairment, or permanent disfigurement. As a result, the primary clinician has the task of determining which lesions require early consultation with a specialist. Although several recent reviews have been published, this clinical report is the first based on input from individuals representing the many specialties involved in the treatment of IH. Its purpose is to update the pediatric community regarding recent discoveries in IH pathogenesis, treatment, and clinical associations and This document is copyrighted and is property of the American to provide a basis for clinical decision-making in the management of IH. Academy of Pediatrics and its Board of Directors. All authors have filed conflict of interest statements with the American Academy of Pediatrics. Any conflicts have been resolved through a process approved by the Board of Directors. The American Academy of Pediatrics has neither solicited nor accepted any commercial involvement in the development of the content of this publication. NOMENCLATURE Clinical reports from the American Academy of Pediatrics benefit from The nomenclature and classification of vascular tumors and expertise and resources of liaisons and internal (American Academy malformations have evolved from clinical descriptions (“strawberry of Pediatrics) and external reviewers. -

Infantile Hemangiomas: Our Current Understanding and Treatment Options
Volume 24 Number 9| September 2018| Dermatology Online Journal || Review 24(9): 1 Infantile hemangiomas: our current understanding and treatment options Yssra S Soliman1 BA, Amor Khachemoune2,3 MD Affiliations: 1Albert Einstein College of Medicine, Bronx, New York, USA, 2State University of New York Downstate, Department of Dermatology, Brooklyn, New York, USA 3Veterans Health Administration, Department of Dermatology, Brooklyn, New York, USA Corresponding Author: Amor Khachemoune MD, FAAD, FACMS, Veterans Affairs Hospital & SUNY Downstate, Dermatology Service, 800 Poly Place, Brooklyn, NY 11209, Tel: 718-630-3725, Fax: 718-630-2881, Email: [email protected] or in visceral organs. Classically, the hemangiomas Abstract display rapid growth during the third month of life, Infantile hemangioma (IH) is the most common followed by slow involution [4]. By 48 months, vascular tumor of infancy, affecting up to 10% of all approximately 90% of cases will have regressed [5]. infants. Our understanding of IH and its management has greatly evolved. The etiology of IH is unclear but This article aims to review the epidemiology, clinical hypoxia is thought to play a key role. Furthermore, presentation, genetics, histopathology, associated GLUT1, IGF2, and HIF-1- syndromes, evolution without treatment, and important mediators. Current management options treatment options of infantile hemangioma. The include active observation, medical treatment, and primary database used was PubMed. We searched surgical intervention. The goals of treatment are , preventing cosmetic disfiguration, psychosocial , distress, and life-threatening complications. Infantile hemangioma should be managed with an individual, f patient-centered approach. Generally, uncompli- -1- cated IH can be observed up to 18 months. However, IH should be treated in the setting of bleeding, Historical note and evolving classification ulceration, functional compromise, or eventual Infantile hemangioma has been reported in the failure to regress. -

Kaposiform Hemangioendothelioma: Current Knowledge and Future Perspectives Yi Ji1*† , Siyuan Chen2*†, Kaiying Yang1, Chunchao Xia3 and Li Li4
Ji et al. Orphanet Journal of Rare Diseases (2020) 15:39 https://doi.org/10.1186/s13023-020-1320-1 REVIEW Open Access Kaposiform hemangioendothelioma: current knowledge and future perspectives Yi Ji1*† , Siyuan Chen2*†, Kaiying Yang1, Chunchao Xia3 and Li Li4 Abstract Kaposiform hemangioendothelioma (KHE) is a rare vascular neoplasm with high morbidity and mortality. The initiating mechanism during the pathogenesis of KHE has yet to be discovered. The main pathological features of KHE are abnormal angiogenesis and lymphangiogenesis. KHEs are clinically heterogeneous and may develop into a life- threatening thrombocytopenia and consumptive coagulopathy, known as the Kasabach-Merritt phenomenon (KMP). The heterogeneity and the highly frequent occurrence of disease-related comorbidities make the management of KHE challenging. Currently, there are no medications approved by the FDA for the treatment of KHE. Multiple treatment regimens have been used with varying success, and new clinical trials are in progress. In severe patients, multiple agents with variable adjuvant therapies are given in sequence or in combination. Recent studies have demonstrated a satisfactory efficacy of sirolimus, an inhibitor of mammalian target of rapamycin, in the treatment of KHE. Novel targeted treatments based on a better understanding of the pathogenesis of KHE are needed to maximize patient outcomes and quality of life. This review summarizes the epidemiology, etiology, pathophysiology, clinical features, diagnosis and treatments of KHE. Recent new concepts and future perspectives for KHE will also be discussed. Keywords: Kaposiform hemangioendothelioma, Kasabach-Merritt phenomenon, Angiogenesis, Lymphangiogenesis, Treatment Introduction the aim of improving our understanding of KHE and pre- Kaposiform hemangioendothelioma (KHE) is a rare vascu- venting mortality and morbidity in severe cases. -

A Capillary Angioma of the Lips: an Extremely Rare Situation in the Oral Cavity
Int. J. Odontostomat., 14(3):354-357, 2020. A Capillary Angioma of the Lips: An Extremely Rare Situation in the Oral Cavity Angioma Capilar de los Labios: Una Situación Extremadamente Rara en la Cavidad Oral Amminou, Loubna1 & El Harti, Karima2 AMMINOU L. & EL HARTI, K. A capillary angioma of the lips: An extremely rare situation in the oral cavity. Int. J. Odontostomat., 14(3):354-357, 2020. ABSTRACT: Hemangiomas are tumors identified by rapid endothelial cell proliferation in early infancy, followed mostly by involution over the time. However, 10-12 % of true hemangiomas don’t involute and require surgical treatment. Hemangiomas are classified on the basis of their histological appearance as capillary, mixed and cavernous. We report a case of capillary angioma in a 13-year old boy who presented with a budding into the lower lip. The lesion was excised and histopathological report confirmed the diagnosis. KEY WORDS: capillary angioma, lips. INTRODUCTION Oral cavity hemangiomas are not commonly most common type, it occurs as a small localized lesion encountered by general dentists. In fact, hemangiomas and has the less-aggressive clinical behavior (da Sil- are rarely seen in the oral cavity especially in the oral va et al., 2014). Its most common presentation is the soft tissue (Handa et al., 2013). port-wine stain. In the soft tissue it can appear as a soft mass, smooth or lobulated, sessile or pedunculated The term “hemangioma” is derived from the and may be seen in any size from a few millimeters to Greek term “hema” denoting “blood,” ‘angio’ denoting several centimeters.