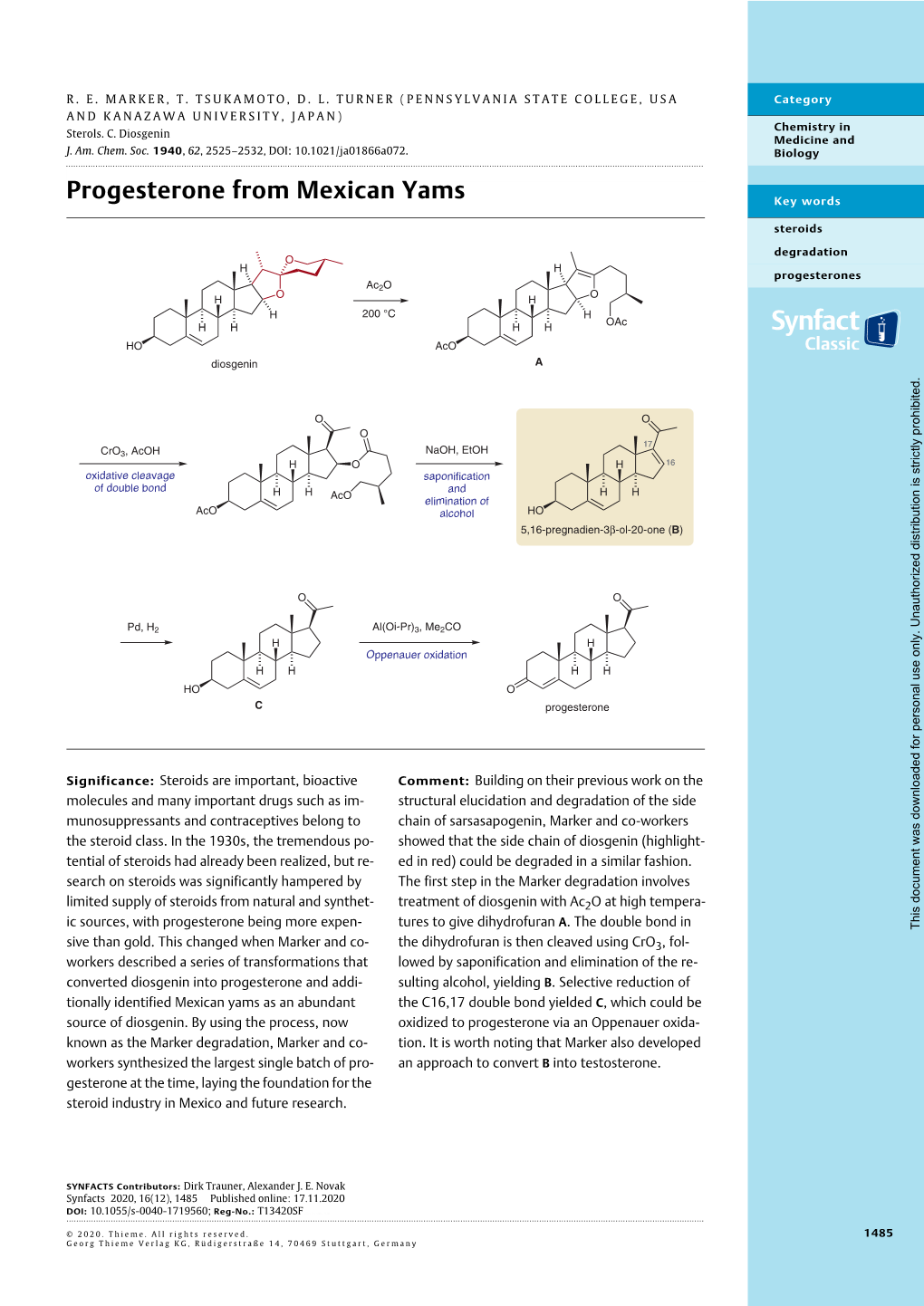
Progesterone from Mexican Yams Key Words
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Saponins from Chinese Medicines As Anticancer Agents
molecules Review Saponins from Chinese Medicines as Anticancer Agents Xiao-Huang Xu 1,†, Ting Li 1,†, Chi Man Vivienne Fong 1, Xiuping Chen 1, Xiao-Jia Chen 1, Yi-Tao Wang 1, Ming-Qing Huang 2,* and Jin-Jian Lu 1,* 1 State Key Laboratory of Quality Research in Chinese Medicine, Institute of Chinese Medical Sciences, University of Macau, Macao, China; [email protected] (X.-H.X.); [email protected] (T.L.); [email protected] (C.M.V.F.); [email protected] (X.C.); [email protected] (X.-J.C.); [email protected] (Y.-T.W.) 2 College of Pharmacy, Fujian University of Traditional Chinese Medicine, Fuzhou 350122, China * Correspondence: [email protected] (M.-Q.H.); [email protected] (J.-J.L.); Tel.: +86-591-2286-1135 (M.-Q.H.); +85-388-224-674 (J.-J.L.) † The authors contribute equally to this work. Academic Editor: Derek J. McPhee Received: 15 August 2016; Accepted: 30 September 2016; Published: 5 October 2016 Abstract: Saponins are glycosides with triterpenoid or spirostane aglycones that demonstrate various pharmacological effects against mammalian diseases. To promote the research and development of anticancer agents from saponins, this review focuses on the anticancer properties of several typical naturally derived triterpenoid saponins (ginsenosides and saikosaponins) and steroid saponins (dioscin, polyphyllin, and timosaponin) isolated from Chinese medicines. These saponins exhibit in vitro and in vivo anticancer effects, such as anti-proliferation, anti-metastasis, anti-angiogenesis, anti-multidrug resistance, and autophagy regulation actions. In addition, related signaling pathways and target proteins involved in the anticancer effects of saponins are also summarized in this work. -

MEDICINAL PLANTS OPIUM POPPY: BOTANY, TEA: CULTIVATION to of NORTH AFRICA Opidjd CHEMISTRY and CONSUMPTION by Loutfy Boulos
hv'IERIGAN BCXtlNICAL COJNCIL -----New Act(uisition~---------l ETHNOBOTANY FLORA OF LOUISIANA Jllll!llll GUIDE TO FLOWERING FLORA Ed. by Richard E. Schultes and Siri of by Margaret Stones. 1991. Over PLANT FAMILIES von Reis. 1995. Evolution of o LOUISIANA 200 beautiful full color watercolors by Wendy Zomlefer. 1994. 130 discipline. Thirty-six chapters from and b/w illustrations. Each pointing temperate to tropical families contributors who present o tru~ accompanied by description, habitat, common to the U.S. with 158 globol perspective on the theory and and growing conditions. Hardcover, plates depicting intricate practice of todoy's ethnobotony. 220 pp. $45. #8127 of 312 species. Extensive Hardcover, 416 pp. $49.95. #8126 glossary. Hardcover, 430 pp. $55. #8128 FOLK MEDICINE MUSHROOMS: TAXOL 4t SCIENCE Ed. by Richard Steiner. 1986. POISONS AND PANACEAS AND APPLICATIONS Examines medicinal practices of by Denis Benjamin. 1995. Discusses Ed. by Matthew Suffness. 1995. TAXQL® Aztecs and Zunis. Folk medicine Folk Medicine signs, symptoms, and treatment of Covers the discovery and from Indio, Fup, Papua New Guinea, poisoning. Full color photographic development of Toxol, supp~. Science and Australia, and Africa. Active identification. Health and nutritional biology (including biosynthesis and ingredients of garlic and ginseng. aspects of different species. biopharmoceutics), chemistry From American Chemical Society Softcover, 422 pp. $34.95 . #8130 (including structure, detection and Symposium. Softcover, isolation), and clinical studies. 223 pp. $16.95. #8129 Hardcover, 426 pp. $129.95 #8142 MEDICINAL PLANTS OPIUM POPPY: BOTANY, TEA: CULTIVATION TO OF NORTH AFRICA OpiDJD CHEMISTRY AND CONSUMPTION by Loutfy Boulos. 1983. Authoritative, Poppy PHARMACOLOGY TEA Ed. -

In Chemistry, Glycosides Are Certain Molecules in Which a Sugar Part Is
GLYCOSIDES Glycosides may be defined as the organic compounds from plants or animal sources, which on enzymatic or acid hydrolysis give one or more sugar moieties along with non- sugar moiety. Glycosides play numerous important roles in living organisms. Many plants store important chemicals in the form of inactive glycosides; if these chemicals are needed, the glycosides are brought in contact with water and an enzyme, and the sugar part is broken off, making the chemical available for use. Many such plant glycosides are used as medications. In animals (including humans), poisons are often bound to sugar molecules in order to remove them from the body. Formally, a glycoside is any molecule in which a sugar group is bonded through its carbon atom to another group via an O-glycosidic bond or an S-glycosidic bond; glycosides involving the latter are also called thioglycosides. The sugar group is then known as the glycone and the non-sugar group as the aglycone or genin part of the glycoside. The glycone can consist of a single sugar group (monosaccharide) or several sugar groups (oligosaccharide). Classification Classification based on linkages Based on the linkage of sugar moiety to aglycone part 1. O-Glycoside:-Here the sugar is combined with alcoholic or phenolic hydroxyl function of aglycone.eg:-digitalis. 2. N-glycosides:-Here nitrogen of amino group is condensed with a sugar ,eg- Nucleoside 3. S-glycoside:-Here sugar is combined with sulphur of aglycone,eg- isothiocyanate glycosides. 4. C-glycosides:-By condensation of a sugar with a cabon atom, eg-Cascaroside, aloin. Glycosides can be classified by the glycone, by the type of glycosidic bond, and by the aglycone. -

Traditional Medicine Research Doi: 10.12032/TMR20210616237
Traditional Medicine Research doi: 10.12032/TMR20210616237 Annual advances of integrative pharmacology in 2020 Ke-Wu Zeng1*, Ming-Yao Gu2* 1State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Beijing 100191, China; 2Department of Cell Biology and Medical Genetics, School of Basic Medical Sciences, Shenzhen University Health Science Center, Shenzhen 518061, China. *Corresponding to: Ke-Wu Zeng, State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, No.38 Xueyuan Road, Haidian District, Beijing 100191, China; E-mail: [email protected]. Ming-Yao Gu, Department of Cell Biology and Medical Genetics, School of Basic Medical Sciences, Shenzhen University Health Science Center, No.1066 Xueyuan Avenue, Nanshan District, Shenzhen 518061, China; E-mail: [email protected]. Highlights This review covers the studies in the year 2020 for pharmacological reports on traditional medicine as well as herb-derived active natural products. Moreover, the pharmacological reports on active natural products against cancers, inflammation, and metabolic diseases were major topics. Tradition This annual integrative pharmacology review includes the reports published in 2020 on bioactive herbal extracts and novel compounds in traditional medicine. Pharmacological reports on traditional herbs as well as their active compounds for anticancer, inflammation, and metabolic diseases occupy dominant positions. Submit a manuscript: https://www.tmrjournals.com/tmr 1 doi: 10.12032/TMR20210616237 REVIEW Abstract Major studies on the pharmacology of traditional herbs as well as active compounds have been introduced in this review over the previous 12 months. This annual integrative pharmacology review includes the reports published in 2020 on bioactive herbal extracts and novel compounds in traditional medicine. -

Print This Article
PEER-REVIEWED ARTICLE bioresources.com GC-MS Characterisation of Sapogenins from Sisal Waste and a Method to Isolate Pure Hecogenin Jener David G. Santos * and Alexsandro Branco ** Five steroidal sapogenins (tigogenin, neotigogenina, hecogenin, gloriogenin, and dehydrohecogenin) were characterised by gas chromatography coupled with mass spectrometry (GC-MS) from a hydrolysed extract of sisal waste. In addition, pure hecogenin, an important raw material for the pharmaceutical industry, was obtained from this waste by selective liquid-liquid extraction of saponins with only hecogenin as aglycone, followed by acid hydrolysis. The yield of pure hecogenin was 460 mg.Kg-1 of sisal waste. Keywords: Agave sisalana; Sisal waste; Extraction; Steroids; Hecogenin Contact information: Laboratory of Phytochemistry, State University of Feira de Santana, 44.036-900 Feira de Santana, Bahia, Brazil; Corresponding authors: *[email protected], **[email protected] INTRODUCTION Steroidal sapogenins are a glycone non-sugar portion of the saponin molecule used for the semi-synthesis of bioactive compounds. Example compounds used in this application include the following: smilagenin, sarsasapogenin, diosgenin, yamogenin, tigogenin, neotigogenin, gloriogenin, gentrogenin, hecogenin, sisalagenin, 9-dehydro- hecogenin, and gitogenin (Agrawal et al. 1985). Among these steroidal sapogenins, diosgenin, sarsasapogenin, and hecogenin are particularly important. The usefulness of hecogenin (Fig. 1) as a synthetic starting material is due to the presence of an oxygen atom in the C-12 position that can be moved to the C-11 position. This makes it possible to introduce the 9-11 double bond required for the syntheses of corticosteroids (Beauvoir 1976). Fig. 1. Chemical structural of hecogenin In the 1940s, steroidal sapogenins achieved great economic importance because of their transformation into pharmaceutically valuable derivatives such as corticosteroids (prednisone, dexamethasone, betamethasone, triamcinolone, and others), sexual hormones, and steroid diuretics (Fernández-Herrera et al. -

Drugs That Changed the World
Drugs That Changed the World Drugs That Changed the World How Therapeutic Agents Shaped Our Lives Irwin W. Sherman CRC Press Taylor & Francis Group 6000 Broken Sound Parkway NW, Suite 300 Boca Raton, FL 33487-2742 © 2017 by Taylor & Francis Group, LLC CRC Press is an imprint of Taylor & Francis Group, an Informa business No claim to original U.S. Government works Printed on acid-free paper Version Date: 20160922 International Standard Book Number-13: 978-1-4987-9649-1 (Hardback) This book contains information obtained from authentic and highly regarded sources. While all reasonable efforts have been made to publish reliable data and information, neither the author[s] nor the publisher can accept any legal respon- sibility or liability for any errors or omissions that may be made. The publishers wish to make clear that any views or opinions expressed in this book by individual editors, authors or contributors are personal to them and do not neces- sarily reflect the views/opinions of the publishers. The information or guidance contained in this book is intended for use by medical, scientific or health-care professionals and is provided strictly as a supplement to the medical or other professional’s own judgement, their knowledge of the patient’s medical history, relevant manufacturer’s instructions and the appropriate best practice guidelines. Because of the rapid advances in medical science, any information or advice on dosages, procedures or diagnoses should be independently verified. The reader is strongly urged to consult the relevant national drug formulary and the drug companies’ and device or material manufacturers’ printed instructions, and their websites, before administering or utilizing any of the drugs, devices or materials mentioned in this book. -

Educación Química, Vol. 1, Núm. 0
PARA QUITARLE EL POLVO Syntex, una historia mexicana La química en la historia, para la enseñanza y su divulgación en el bachillerato Felipe León Olivares1 Resumen de no disponer de hormonas en cantidad y calidad El presente trabajo explica la importancia de las suficiente, generaba un problema ya que la produc- aportaciones de la investigación científica lograda en ción era limitada y los precios muy elevados, en gran México en el campo de la Química por Syntex, medida por los procesos complejos con rendimien- donde se realizó una verdadera revolución mundial tos bajos que usaban las compañías europeas. A en la síntesis orgánica de las hormonas esteroides. pesar de esta situación, mantenían el control tecno- De esta manera, se considera importante su divulga- lógico dominando el mercado internacional de estos ción en el bachillerato, como una experiencia que productos. Por su parte, las sucursales europeas en proyecta interés al estudio de la química a las nuevas Estados Unidos tuvieron un especial éxito comercial generaciones de estudiantes. motivando a éstas a una mayor actividad de investi- gación. Por ejemplo, la Upjohn y la Parke-Davis, Introducción fomentaron una extensa investigación a través de un El presente estudio plantea la alternativa de la divul- programa de becas. Así fue que el químico Russell gación de las aportaciones de la investigación cien- E. Marker, de la Universidad Estatal de Pennsylva- tífica realizada en México en el campo de la Quími- nia, inició sus estudios sobre las hormonas esteroides ca. Ejemplos como el de ‘‘Andrés Manuel del Río’’ (Lehmann, et al., 1973:196). (Carrera, 1956:5), ‘‘Vicente Ortigosa’’ (Chamizo, Marker planteó que el punto clave en la indus- 1999:138), y el de la empresa ‘‘Hojalata y Lámina de tria de las hormonas esteroides estaba en la materia Monterrey’’ (Garritz, 1993:36), entre otros aconteci- prima; con esta hipótesis prestó atención a las plantas mientos dignos de mención, deben ser conocidos como fuente barata y abundante de hormonas. -

Repeated Evolution of Cytochrome P450-Mediated Spiroketal Steroid Biosynthesis in Plants
Repeated evolution of cytochrome P450- mediated spiroketal steroid biosynthesis in plants The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation Christ, Bastien et al. "Repeated evolution of cytochrome P450- mediated spiroketal steroid biosynthesis in plants." Nature communications 10 (2019): 1038 © 2019 The Author(s) As Published 10.1038/s41467-019-11286-7 Publisher Springer Science and Business Media LLC Version Final published version Citable link https://hdl.handle.net/1721.1/124710 Terms of Use Creative Commons Attribution 4.0 International license Detailed Terms https://creativecommons.org/licenses/by/4.0/ ARTICLE https://doi.org/10.1038/s41467-019-11286-7 OPEN Repeated evolution of cytochrome P450-mediated spiroketal steroid biosynthesis in plants Bastien Christ1,6,7, Chengchao Xu1,7, Menglong Xu1,7, Fu-Shuang Li1, Naoki Wada 2, Andrew J. Mitchell1, Xiu-Lin Han3, Meng-Liang Wen3, Makoto Fujita2,4 & Jing-Ke Weng 1,5 Diosgenin is a spiroketal steroidal natural product extracted from plants and used as the single most important precursor for the world steroid hormone industry. The sporadic 1234567890():,; occurrences of diosgenin in distantly related plants imply possible independent biosynthetic origins. The characteristic 5,6-spiroketal moiety in diosgenin is reminiscent of the spiroketal moiety present in anthelmintic avermectins isolated from actinomycete bacteria. How plants gained the ability to biosynthesize spiroketal natural products is unknown. Here, we report the diosgenin-biosynthetic pathways in himalayan paris (Paris polyphylla), a monocot med- icinal plant with hemostatic and antibacterial properties, and fenugreek (Trigonella foenum–graecum), an eudicot culinary herb plant commonly used as a galactagogue. -

United States Patent Office W
w 2,870,143 United States Patent Office Patented Jan. 20, 1959 2 2,870,143 the structure of the parent sapogenin and that heating beyond the point of complete conversion gives lower PROCESS FOR CONVERSION OF STEROIDAL yields of the pseudosapogenins. In particular, differ SAPOGENINS TOPSEUDOSAPOGENNS ences in the spiroketal side chain configuration of Sapog Monroe E. Wall, Oreland, and Samuel Serota, Phila eninsis one factor which leads to significant differences delphia, Pa., assignors to the United States of America in the rate of conversion of the sapogenin to pseudo as represented by the Secretary of Agriculture sapogenin. In the accompanying table (Table I) each No Drawing. Application January 22, 1958 pair of sapogenins is identical except for isomerism in the spiroketal side chain. The site of isomerism, now Serial No. 710,588 10 considered to be at C25 (cf. M. E. Wall, Experientia 11, i8. Claims. (Cl. 260-239.55) 340 (1955), for a review of pertinent literature), is im material to the present invention. The significance of (Granted under Title 35, U.S. Code (1952), sec. 266) the data in Table I is that in each pair of normal and A non-exclusive, irrevocable, royalty-free license in the iso sapogenins, the normal isomer is converted to its re invention herein described, throughout the world for all 5 spective pseudosapogenin much more rapidly than is the purposes of the United States Government, with the power iso analogue. to grant sublicenses for such purposes, is hereby granted TABLE. I.-CONVERSION OF NATURAL SAPOGENINS TO to the Government of the United States of America. -

International Historic Chemical Landmark Acclaims Success of Mexican Steroid Industry and a U.S
Journal of the Mexican Chemical Society ISSN: 1870-249X [email protected] Sociedad Química de México México Raber, Linda Steroid industry honored. International historic chemical landmark acclaims success of mexican steroid industry and a U.S. chemist who made it possible Journal of the Mexican Chemical Society, vol. 43, núm. 6, noviembre-diciembre, 1999, pp. 235-237 Sociedad Química de México Distrito Federal, México Available in: http://www.redalyc.org/articulo.oa?id=47543610 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Revista de la Sociedad Química de México, Vol. 43, Núm. 6 (1999) 235-237 Noticias Steroid Industry Honored† International Historic Chemical Landmark Acclaims Success of Mexican Steroid Industry and a U.S. Chemist Who Made it Possible Linda Raber American Chemical Society 1155-16th St., N.W, Washington, D.C. 20036. U.S.A. “There are more stories told about Russell Marker than any he founded in Mexico City with Emeric Somlo and Federico other chemist. Although perhaps many of these stories are A. Lehmann. apocryphal, they are so fascinating that most of us cannot bear “This low-cost progesterone eventually became the pre- to stop repeating them. This is the oral history of our profes- ferred precursor in the industrial preparation of the anti- sion that we pass to our colleagues and our students. They are inflammatory drug cortisone. In 1951, Syntex researchers syn- the campfire stories that bind our profession together” – thesized the first useful oral contraceptive from Marker’s start- Steven M. -

Aerobic Microbial Transformations of Sterols and Steroids, a Personal Approach Bernardo Servín-Massieu CONTENTS Overview the Fo
Aerobic Microbial Transformations of Sterols and Steroids, a personal Approach Bernardo Servín-Massieu CONTENTS Overview The following article has the purpose to publish a malicious bias to give the impression that the development of the steroid industry in Mexico was only the contribution from alumni from the National School of Chemistry Sciences and the Chemistry Institute, both belonging to the National Autonomous University of Mexico in Mexico City promoted by the Mexican Chemistry Society. Not at all, alumni from the National School of Biological Sciences from the National Polytechnic Institute in Mexico City made a substantial contribution to the aerobic microbial transformation of, initially steroid molecules and afterwards in the microbial transformation of sterols. In the 1940’s of the XX century Russell E. Marker designed the transformation of diosgenin to progesterone using wild yams collected in the Metlac ravine near the town of Orizaba in the state of Veracruz about 200 miles east Mexico City. It is known as Marker’s degradation being the kick-off of a new industry in Mexico. These yams were from Dioscorea mexicana known by the local inhabitants as “cabeza de negro” negro’s head, a vine that grows wild in moist subtropical forest in Veracruz, Puebla, Chiapas and Oaxaca states, further along Marker discovered that “Barbasco” Dioscorea compositae and Dioscorea floribunda contained higher contents of diosgenin. Marker consulted the Mexico City yellow pages after checking at the Hotel Geneve, London Street, Pink Zone and found an entry “Laboratorios Hormona, S.A.”, he called Federico Lehmann and agreed to meet. It can be said that the steroid industry boom in Mexico was indirectly due to Adolph Hitler’s Nazism and Francisco Franco’s Fascism that respectively caused the Jewish diaspora in Germany and “refugiados” after the socialist civil war in Spain. -

Anticancer Properties of Phytochemicals Present in Medicinal Plants of North America
Chapter 6 Anticancer Properties of Phytochemicals Present in Medicinal Plants of North America Wasundara Fernando and H. P. Vasantha Rupasinghe Additional information is available at the end of the chapter http://dx.doi.org/10.5772/55859 1. Introduction Cancer is one of the most severe health problems in both developing and developed countries, worldwide. Among the most common (lung, stomach, colorectal, liver, breast) types of cancers, lung cancer has continued to be the most common cancer diagnosed in men and breast cancer is the most common cancer diagnosed in women. An estimated 12.7 million people were diagnosed with cancer across the world in 2008, and 7.6 million people died from the cancer during the same year [1]. Lung cancer, breast cancer, colorectal cancer and stomach cancer accounted for two-fifths of the total cases of cancers diagnosed worldwide [1]. More than 70% of all cancer deaths occurred in low- and middle-income countries. Deaths due to cancer are projected to continuously increase and it has been estimated that there will be 11.5 million deaths in the year 2030 [1] and 27 million new cancer cases and 17.5 million cancer deaths are projected to occur in the world by 2050 [2]. According to Canadian cancer statistics, issued by the Canadian Cancer Society, it is estimated that 186,400 new cases of cancer (excluding 81,300 non-melanoma skin cancers) and 75,700 deaths from cancer will occur in Canada in 2012 [1]. The lowest number of incidences and mortality rate is recorded in British Columbia. Both incidence and mortality rates are higher in Atlantic Canada and Quebec [3].