Surgical Management of Male Infertility
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Spring 06 2 27.Qxp
University of California, Irvine School of Medicine Spring 2006 UCI Medical Center Department of Urology 101 THE CITY DRIVE SOUTH ORANGE, CA 92868-5395 N E W S L E T T E R Re-United: The facts about vasectomy reversal "When a man consents to undergo a vasectomy, he is usually instructed that the procedure should be considered Aaron Spitz, MD to be permanent and irreversible.... Nonetheless, even the Assistant Clinical Professor Male Reproductive Medicine most insightful, thoughtful decision can ultimately prove and Surgery wrong. When that decision is a vasectomy, a man may still change his mind." re you considering a vasec- tomy. Therefore, before undergoing a tomy reversal? Thousands vasectomy, a man should be as sure as A of men undergo vasectomy possible that he is finished having chil- each year as a permanent means of dren. Nonetheless, even the most birth control, yet for some of these men insightful, thoughtful decision can ulti- life brings unexpected turns, which mately prove wrong. When that deci- leads them to change their mind. For sion is a vasectomy, a man may still Epididymo-vasostomy some, there is a strong desire to have change his mind. sperm travel from the testicle to the another child a few years later. For urethra. It feels like a piece of under- others, there may be a tragic loss of a What is a vasectomy? cooked spaghetti in each side of the child. For many men, a new marriage To understand the vasectomy reversal, scrotum. The sperm are produced in brings a new opportunity for creating a it is important to understand the vasec- the testicle, and then they exit out the family. -

Vasectomy Reversal (VR)
Vasectomy Reversal (VR) ! Need to know Please read this before your Vasectomy Reversal Preparing for the VR • Do not eat or drink after midnight if your operation is in the morning and not after 7.00am if it is in the afternoon. • Please shave the hair at the front and sides of the scrotum from the base of the penis down. You do not need to shave the pubic hair • Take supportive underpants (not your best) or a jockstrap into the hospital with you and to theatre to wear after the operation. • Arrange a week off work. • Avoid intercourse for two weeks after the operation and heavy lifting for four weeks. REF 0000.0 – 12/15 What to expect during the VR • An infection of the scrotum rarely occurs but if it does, • An incision is placed on each side of the scrotum that will present a few days after the surgery and is apparent are a little larger than those used for the vasectomy. because the pain becomes worse rather than better and the scrotum becomes red. • The vas is a very small muscular tube with a 2mm outer diameter and an inner diameter through which If you experience any of the above complications or sperm flow (the lumen), of less than 1/2mm. Since the have any other problem occur after your VR, please structure is so small, the stitches must be placed very call the Clinic or your Surgeon if outside clinic exactly so that there is minimal scarring. Too much hours. scarring can cause the lumen of the vas to close and the procedure to fail. -

The History of Microsurgery in Urological Practice
Chen-1 The History of Microsurgery in Urological Practice Mang L. Chen1, Gregory M. Buncke2 and Paul J. Turek3 1G.U. Recon, San Francisco, CA, 94114 2The Buncke Clinic, San Francisco, CA 94114 3The Turek Clinic, Beverly Hills, CA 90210 Correspondence to: Mang Chen, MD G.U. Recon 45 Castro St, Suite 111 San Francisco, CA 94114 Tel: 415-481-3980 Email: [email protected] Chen-2 Abstract Operative microscopy spans all surgical disciplines, allowing human dexterity to perform beyond direct visual limitations. Microsurgery started in otolaryngology, became popular in reconstructive microsurgery, and was then adopted in urology. Starting with reproductive tract reconstruction of the vas and epididymis, microsurgery in urology now extends to varicocele repair, sperm retrieval, penile transplantation and free flap phalloplasty. By examining the peer reviewed and lay literature this review discusses the history of microsurgery and its subsequent development as a subspecialty in urology. Keywords: urology, microsurgery, phalloplasty, vasovasostomy, varicocelectomy Chen-3 I. Introduction Microsurgery has been instrumental to surgical advances in many medical fields. Otolaryngology, ophthalmology, gynecology, hand and plastic surgery have all embraced the operating microscope to minimize surgical trauma and scar and to increase patency rates of vessels, nerves and tubes. Urologic adoption of microsurgery began with vasectomy reversals, testis transplants, varicocelectomies and sperm retrieval and has now progressed to free flap phalloplasties and penile transplantation. In this review, we describe the origins of microsurgery, highlight the careers of prominent microsurgeons, and discuss current use applications in urology. II. Birth of Microsurgery 1) Technology The birth of microsurgery followed from an interesting marriage of technology and clinical need. -

Parviz K. Kavoussi, M.D., F.A.C.S. Reproductive Urologist
PARVIZ K. KAVOUSSI, M.D., F.A.C.S. REPRODUCTIVE UROLOGIST www.AustinFertility.com www.AustinVasecomyCenter.com www.WestlakeIVF.com www.AustinMensHealth.com WESTLAKE LOCATION: 300 BEARDSLEY LANE Building B, SUITE 200 AUSTIN, TX 78746 SOUTH AUSTIN LOCATION: 4303 JAMES CASEY, SUITE B AUSTIN, TEXAS 78745 ROUND ROCK LOCATION: 7700 CAT HOLLOW DRIVE, SUITE 106 ROUND ROCK, TEXAS 78681 Phone: (512) 444-1414 EXT 2 Fax: (512) 441-1202 POSTDOCTORAL TRAINING THE UNIVERSITY OF VIRGINIA HEALTH SCIENCES CENTER. Charlottesville, Virginia. Fellowship- Reproductive Urology/Andrology – Male Infertility, Sexual Medicine, and Microsurgery (Vasectomy Reversals/Sperm Retrievals). National Institute of Health funded fellow. July 2008-June 2010. BAYLOR SCOTT & WHITE MEMORIAL HOSPITAL AND HEALTH SCIENCES CENTER. Temple, Texas. Residency- Urology- July 2004-June 2008. General Surgery July 2002-June 2004 EDUCATION THE UNIVERSITY OF TEXAS MEDICAL BRANCH AT GALVESTON. Galveston, Texas. Doctor of Medicine. August 1998-May 2002. BAYLOR UNIVERSITY. Waco, Texas. Bachelor of Arts, degree in Biology. August 1994-May 1998. BOARD CERTIFICATION The American Board of Urology ACADEMIC APPOINTMENTS • Adjunct Assistant Professor. Department of Urology. University of Texas Health Sciences Center at San Antonio. September 2015-present. • Adjunct Assistant Professor. Department of Psychology, Division of Neuroendocrinology and Motivation. University of Texas at Austin. March 2013- present. SPECIALTY CREDENTIALS RAMSES. Credentialed in Robot Assisted Microsurgery in Urology/Robot Assisted Vasectomy Reversals by RAMSES (Robot Assisted Microsurgical and Endoscopic Society). Winter Haven Hospital & University of Florida. April 2011. LABORATORY EXPERIENCE • Basic science laboratory research in the Department of Urology at University of Virginia funded via NIH training grant. July 2008-June 2010. • Non-human primate surgery at the Michael E. -

UROLOGY AUA Honors Dr
IN THIS ISSUE THE UNIVERSITY OF ILLINOIS HOSPITAL & HEALTH SCIENCES SYSTEM Lawrence Ross Honored 1 New Residents and Fellows 2 UROLOGY AUA Honors Dr. Prins 4 5 Summer 2020 Urology Graduates Look Ahead Publications 7 DISTINGUISHED CONTRIBUTION AWARD— FROM LAWRENCE S. ROSS, MD DR. NIEDERBERGER Welcome to the Lawrence S. Ross, MD, Clarence C. Saelhof Professor 2020 issue. Emeritus at UI Urology, has received many honors during his 50+ year medical career: the Distinguished Since we last published, a lot Reproductive Urology Award from the Society has happened for the Study of Male Reproduction in 2012 and in the world— a Distinguished Service Award from the Chicago notably the onset Urological Society last year, among many others. of the COVID-19 pandemic, which This year, Dr. Ross added to his list of honors when the has created American Urological Association (AUA) named him a upheaval on so recipient of its Distinguished Contribution Award, given many levels. Like all of you, we have for “contributions to the field of reproductive medicine put public health and the safety of and for many years of dedicated service to the AUA.” our patients, colleagues, and families first. We hope you and those close Dr. Ross, a past president of the AUA, who also served on the association’s board to you are well and safe. for five years, is humbled by the recognition. “It is a very big thing in a urological career, even if you’ve been president, to be recognized like this. This is a singular In the article in this issue about honor in one’s academic career.” receiving a Presidential Citation from the American Urological Association, The Distinguished Contribution Award is given to only a few AUA members every Gail S. -

Successful Unilateral Vasectomy Reversal in a Lion (Panthera Leo)
Open Veterinary Journal, (2019), Vol. 9(4): 322–326 ISSN: 2226-4485 (Print) Case Report ISSN: 2218-6050 (Online) DOI: http://dx.doi.org/10.4314/ovj.v9i4.8 Submitted: 30/04/2019 Accepted: 14/10/2019 Published: 29/11/2019 Successful unilateral vasectomy reversal in a lion (Panthera leo) Marcelo Marconi1*, José Manuel de la Torre2, Cristian Palma3, Hector Gallegos1, Evelyn Soto4, Sebastián Celis5, Camila de la Torre5, Carolina Ortiz5, Alberto Duarte5 and Ignacio Idalsoaga5 1Andrology Unit, Department of Urology, Pontificia Universidad Catolica, Santiago, Chile 2Department of Urology, El Carmen Hospital, Santiago, Chile 3Department of Urology, Clinica Las Condes, Santiago, Chile 4Andromed, Santiago, Chile 5Department of Veterinary Medicine, Buin Zoo, Buin, Chile Abstract Background: In 2016, the veterinarian team of Buin Zoo in Chile decided to try to increase the lion population. At that time, the zoo had three lions; two females and one male. The 9-year-old male had been vasectomized 5 years ago at the same institution for birth control. Considering the fact that in humans, vasectomy reversal has excellent reproductive outcomes, a team of human urologists, highly experienced in vasectomy reversal was contacted to perform the procedure. Case description: Surgery was performed on June 16, 2016 under general anesthesia, with the vasectomy site accessed through the previous scar localized in the lower groin. After opening the skin, dartos and tunica vaginalis, we were able to identify the previous vasectomy site. After liberating both vas ends and checking for permeability, a microsurgical anastomosis (magnification 25×) was performed. The surgery took 80 minutes with minimal bleeding, and no surgical complications were observed. -

Microsurgical Vasectomy Reversal: Results and Predictors of Success
9REVUE Andrologie 2005, 15, N~ 167-171 Microsurgical vasectomy reversal : results and predictors of success Gert R. DOHLE, Marij SMIT Andrology unit, Department of Urology, Erasmus University Medical Centre, Rotterdam, The Netherlands ABSTRACT successfully. The results of vasectomy reversal procedu- res can be improved substantially if the surgeon is able to perform a vaso-epididymostomy in cases of a secondary Microsurgical vasectomy reversal is a challenge for the epididymal obstruction, occurring in about 25% of men physician but successful treatment depends on the expe- with an interval of more than 10 years. rience and skills of the surgeon. Fertility can often be res- tored, thus avoiding the need for artificial reproductive techniques. Also, the surgical procedures can be combi- Key words : male infertility, microsurgery, vasectomy rever- ned with sperm aspiration and cryopreservation, to be sal, vasoepididymostemy used for Intracytoplasmic sperm injection (ICSI) in cases of surgical failure. We describe the results of 217 vasova- sostomy procedures, with special emphasis on recent technical refinements and prognostic indicators. I. INTRODUCTION Between 1998 and 2002 we performed 217 vasovasosto- my-procedures in an outpatient clinic setting. Microsurgery in urology is mainly applied in obstructive Refertilisation was successful in 76.5%, spontaneous pre- male infertility and varicocele repair. Other indications are gnancy occurred in 42% of the couples after a follow-up vascular erectile dysfunction and penile or testicular vas- of at least 1 year. The main prognostic factors determi- cular trauma. Obstructions of the male genital tract repre- ning the outcome of the surgery was the interval between sent 5-10% of the causes of male infertility and in 70-80% vasectomy and refertilisation and the age of the female of these men surgical repair can be performed [11]. -

Male Infertility
Guidelines on Male Infertility A. Jungwirth, T. Diemer, G.R. Dohle, A. Giwercman, Z. Kopa, C. Krausz, H. Tournaye © European Association of Urology 2012 TABLE OF CONTENTS PAGE 1. METHODOLOGY 6 1.1 Introduction 6 1.2 Data identification 6 1.3 Level of evidence and grade of recommendation 6 1.4 Publication history 7 1.5 Definition 7 1.6 Epidemiology and aetiology 7 1.7 Prognostic factors 8 1.8 Recommendations on epidemiology and aetiology 8 1.9 References 8 2. INVESTIGATIONS 9 2.1 Semen analysis 9 2.1.1 Frequency of semen analysis 9 2.2 Recommendations for investigations in male infertility 10 2.3 References 10 3. TESTICULAR DEFICIENCY (SPERMATOGENIC FAILURE) 10 3.1 Definition 10 3.2 Aetiology 10 3.3 Medical history and physical examination 11 3.4 Investigations 11 3.4.1 Semen analysis 11 3.4.2 Hormonal determinations 11 3.4.3 Testicular biopsy 11 3.5 Conclusions and recommendations for testicular deficiency 12 3.6 References 12 4. GENETIC DISORDERS IN INFERTILITY 14 4.1 Introduction 14 4.2 Chromosomal abnormalities 14 4.2.1 Sperm chromosomal abnormalities 14 4.2.2 Sex chromosome abnormalities 14 4.2.3 Autosomal abnormalities 15 4.3 Genetic defects 15 4.3.1 X-linked genetic disorders and male fertility 15 4.3.2 Kallmann syndrome 15 4.3.3 Mild androgen insensitivity syndrome 15 4.3.4 Other X-disorders 15 4.4 Y chromosome and male infertility 15 4.4.1 Introduction 15 4.4.2 Clinical implications of Y microdeletions 16 4.4.2.1 Testing for Y microdeletions 17 4.4.2.2 Y chromosome: ‘gr/gr’ deletion 17 4.4.2.3 Conclusions 17 4.4.3 Autosomal defects with severe phenotypic abnormalities and infertility 17 4.5 Cystic fibrosis mutations and male infertility 18 4.6 Unilateral or bilateral absence/abnormality of the vas and renal anomalies 18 4.7 Unknown genetic disorders 19 4.8 DNA fragmentation in spermatozoa 19 4.9 Genetic counselling and ICSI 19 4.10 Conclusions and recommendations for genetic disorders in male infertility 19 4.11 References 20 5. -
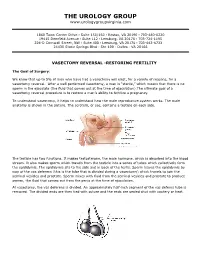
Vasectomy Reversal-Restoring Fertility
THE UROLOGY GROUP www.urologygroupvirginia.com 1860 Town Center Drive · Suite 150/160 · Reston, VA 20190 · 703-480-0220 19415 Deerfield Avenue · Suite 112 · Leesburg, VA 20176 · 703-724-1195 224-D Cornwall Street, NW · Suite 400 · Leesburg, VA 20176 · 703-443-6733 24430 Stone Springs Blvd · Ste 100 · Dulles · VA 20166 VASECTOMY REVERSAL -RESTORING FERTILITY The Goal of Surgery: We know that up to 5% of men who have had a vasectomy will elect, for a variety of reasons, for a vasectomy reversal. After a well performed vasectomy, a man is “sterile,” which means that there is no sperm in the ejaculate (the fluid that comes out at the time of ejaculation).The ultimate goal of a vasectomy reversal procedure is to restore a man’s ability to fertilize a pregnancy. To understand vasectomy, it helps to understand how the male reproductive system works. The male anatomy is shown in the picture. The scrotum, or sac, contains a testicle on each side. The testicle has two functions. It makes testosterone, the male hormone, which is absorbed into the blood stream. It also makes sperm which travels from the testicle into a series of tubes which collectively form the epididymis. The epididymis sits to the side and in back of the testis. Sperm leaves the epididymis by way of the vas deferens (this is the tube that is divided during a vasectomy) which travels to join the seminal vesicles and prostate. Sperm mixes with fluid from the seminal vesicles and prostate to produce semen, the fluid that comes out from the penis at the time of ejaculation. -

Efficacy of Vasectomy Reversal According to Patency for The
International Journal of Impotence Research (2012) 24, 202 -- 205 & 2012 Macmillan Publishers Limited All rights reserved 0955-9930/12 www.nature.com/ijir ORIGINAL ARTICLE Efficacy of vasectomy reversal according to patency for the surgical treatment of postvasectomy pain syndrome JY Lee1, JS Chang1, SH Lee1, WS Ham1, HJ Cho2,TKYoo2, KS Lee3, TH Kim3, HS Moon4, HY Choi4 and SW Lee4 This study was conducted to assess outcomes (according to patency) of vasectomy reversal (VR) in qualified patients with postvasectomy pain syndrome (PVPS). A total of 32 patients with PVPS undergoing VR between January 2000 and May 2010 were examined retrospectively. Of these, 68.8% (22/32) completed a study questionnaire, either onsite at the outpatient clinic or via telephone interview. Preoperative clinical findings, preoperative and postoperative visual analogue scale (VAS) pain scores, patency and pregnancy rate and overall patient satisfaction were analyzed. For the latter, a four-point rating of (1) cure, (2) improvement, (3) no change or (4) recurrence was used. The mean age was 45.09±4.42 years and the mean period of follow-up was 3.22 years (0.74--7.41). Patency rates were 68.2% (15/22) and pregnancy rates were 36.4% (8/22). The mean VAS was 6.64±1.00 preoperatively and 1.14±0.71 postoperatively (Po0.001). The difference in the mean preoperative and postoperative VAS was 6.00±1.25 (4--8) in the patency group and 4.43±0.98 (3--6) in the no patency group (P ¼ 0.011). A significant difference in procedural satisfaction with surgical outcome was observed between patency and no patency groups (P ¼ 0.014). -

REVISED 5TH EDITION VERSION 2.0 (2020) with Page No Watermark.Xlsx
MALAYSIAN MEDICAL ASSOCIATION MALAYSIAN MEDICAL ASSOCIATION MEDICAL PROCEDURES AND SERVICES NOMENCLATURE (MPSN®) AND RELATIVE VALUES GUIDELINES FOR MEDICAL PRACTITIONERS IN MALAYSIA COPY RIGHT 2020 FIRST EDITION --- 1987 SECOND EDITION --- 1992 THIRD EDITION --- 1997 FOURTH EDITION --- 2002 FIFTH EDITION Version 1.0 _ 2008 Version 1.1. _ 2019 Version 2.0. _ 2020 COPY RIGHT NOTICE WARNING: The doing of an unauthorized act in relation to copyright work may result in both a civil claim for damages and criminal prosecution The Malaysian Medical Association (hereinafter referred to as MMA) published the Schedule of Fees in 1987,1992,1997 and 2002. This 2008 Edition has been renamed as Medical Procedures and Services Nomenclature and Schedule of Relative Values (hereinafter referred to as MPSN®) and is protected by copyright and is the property of the MMA as were the previous editions of the Fee Schedule. The 2008 MPSN® is produced by kind permission of materials from the BUPA Schedule of Procedures (1995) of the British United Provident Association Ltd. London, United Kingdom. The BUPA Schedule of Procedures (1995) is based on the UK Office of Population Censuses and Surveys. Classification of Operations and Surgical Procedures 4th Edition © Crown Copyright 1987 and has been used by kind permission of the Controller of Her Majesty’s Stationery Office. In addition to the above, the MPSN® (2008) is the result of work developed by the Health Insurance Committee (HIC) of the MMA. Advice and suggestions by a large number of medical organisations and societies were taken into account. These were via discussions, consultations, written feedback and review of similar fees guidelines published by sister bodies from within and outside the country. -

The Urology Program at Maine Medical Center
The Urology Program at Maine Medical Center A MaineHealth Member Maine Medical Center’s Urologic Conditions Treated Urology Program Specific conditions treated include: • Cancer of the adrenal gland, bladder, kidney, prostate, penis, and testicles The comprehensive urology program at Maine Medical Center (MMC) ensures access to high quality and leading-edge urologic treatment today, • Enlarged prostate, or benign prostatic hyperplasia (BPH) while planning to meet our communities’ future needs. Our urologists • Erectile dysfunction evaluate and manage a full range of adult and pediatric urologic diseases and • Hematuria, or blood in the urine disorders. Patients are provided with exceptional care close to home at both Maine Medical Center and its Maine Medical Partners – Urology office. • Hydrocele/Spermatocele • Kidney stone disease • Male infertility What is Urology? • Neurogenic bladder Urology is a medical and surgical specialty involving the diagnosis and • Overactive bladder treatment of diseases and disorders of the male and female urinary tract • Pediatric urologic problems, including: and the male reproductive organs in both adults and children. – Vesico-ureteral reflux – UPJ obstruction – Penile/urethral anomalies – Voiding dysfunction – Undescended testicle • Pelvic floor disorders • Peyronie’s disease • Urethral stricture • Urinary incontinence, male and female • Urinary tract infections • Varicocele • Vasectomy • Vesicoureteral reflux or a backflow of urine into the ureter • Voiding dysfunction or abnormal bladder emptying Please see our website for additional conditions treated. 1 12 Urologists with Outstanding Milestones and Accolades Experience and Expertise An Impressive Roster of Firsts Maine Medical Center has the largest and most experienced urologic The urologic specialists at MMC have achieved many clinical firsts team in northern New England.