01 Coverpage
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Tiger Reserves in India 2021 (As on July 2021)
TIGER RESERVES IN INDIA 2021 (AS ON JULY 2021) Updated List of Tiger Reserves in India 2021 (as on July 2021) Dear Champions, preparing for competitive exams must know the Updated list of Tiger Reserves in India which is helps to crack the upcoming exams likes SBI Clerk, RRB PO, RRB Assistants, IBPS PO, IBPS Clerk, SSC exams, TNPSC, etc. Here we give the complete list of Tiger Reserves in India as per July 31, 2021. The Tiger Reserves of India were set up in 1973, which is governed by Project Tiger. The Project Tiger administrated by the National Tiger Conservation Authority (NCTA) which was launched in 2005. NCTA is statutory body under the Ministry of Environment, Forest and Climate Change. NCTA is under sec 38 V (1) of the Wildlife (Protection) Act, 1972 which is amended Wildlife (Protection) Amendment Act, 2006. As per July 31, 2021 there are 52 Tiger Reserves in India. Recently Srivilliputhur-Megamalai Tiger Reserve (Tamil Nadu) and Ramgarh Vishdhari Tiger Reserve (Rajasthan) are declared as 51st and 52nd Tiger Reserves of India respectively. List of Tiger Reserves in India 2021 State-wise Andhra Pradesh (1) ✓ Nagarjunsagar Srisailam Tiger Reserve (1982–83) Assam (4) ✓ Manas Tiger Reserve (1973-74) ✓ Nameri Tiger Reserve (1999–2000) ✓ Kaziranga Tiger Reserve (2008–09) ✓ Orang Tiger Reserve (2016) Arunachal Pradesh (3) ✓ Namdapha Tiger Reserve (1982–83) ✓ Pakke or Pakhui Tiger Reserve (1999–2000) ✓ Kamlang Tiger Reserve (2016) Bihar (1) ✓ Valmiki Tiger Reserve (1989–90) Chhattisgarh (3) ✓ Indravati Tiger Reserve (1982–83) ✓ Udanti-Sitanadi Tiger Reserve (2008–09) ✓ Achanakmar Tiger Reserve (2008–09) Jharkhand (1) ✓ Palamau Tiger Reserve (1973-74) F o l l o w u s : YouTube, Website, Telegram, Instagram, Facebook. -

Pench Tiger Reserve: Maharashtra
Pench Tiger Reserve: Maharashtra drishtiias.com/printpdf/pench-tiger-reserve-maharashtra Why in News Recently, a female cub of 'man-eater' tigress Avni has been released into the wild in the Pench Tiger Reserve (PTR) of Maharashtra. Key Points About: It is located in Nagpur District of Maharashtra and named after the pristine Pench River. The Pench river flows right through the middle of the park. It descends from north to south, thereby dividing the reserve into equal eastern and western parts. PTR is the joint pride of both Madhya Pradesh and Maharashtra. The Reserve is located in the southern reaches of the Satpura hills in the Seoni and Chhindwara districts in Madhya Pradesh, and continues in Nagpur district in Maharashtra as a separate Sanctuary. It was declared a National Park by the Government of Maharashtra in 1975 and the identity of a tiger reserve was granted to it in the year 1998- 1999. However, PTR Madhya Pradesh was granted the same status in 1992-1993. It is one of the major Protected Areas of Satpura-Maikal ranges of the Central Highlands. It is among the sites notified as Important Bird Areas (IBA) of India. The IBA is a programme of Birdlife International which aims to identify, monitor and protect a global network of IBAs for conservation of the world’s birds and associated diversity. 1/3 Flora: The green cover is thickly spread throughout the reserve. A mixture of Southern dry broadleaf teak forests and tropical mixed deciduous forests is present. Shrubs, climbers and trees are also frequently present. -
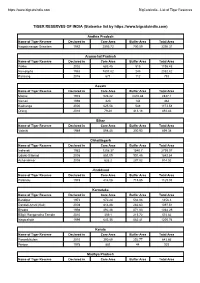
TIGER RESERVES of INDIA (Statewise List By
https://www.bigcatsindia.com BigCatsIndia - List of Tiger Reserves TIGER RESERVES OF INDIA (Statewise list by https://www.bigcatsindia.com) Andhra Pradesh Name of Tiger Reserve Declared in Core Area Buffer Area Total Area Nagarjunsagar Srisailam 1982 2595.72 700.59 3296.31 Arunachal Pradesh Name of Tiger Reserve Declared in Core Area Buffer Area Total Area Pakke 2002 683.45 515 1198.45 Namdapha 1983 1807.82 245 2052.82 Kamlang 2016 671 112 783 Assam Name of Tiger Reserve Declared in Core Area Buffer Area Total Area Manas 1973 526.22 2310.88 2837.1 Nameri 1998 320 144 464 Kaziranga 2006 625.58 548 1173.58 Orang 2016 79.28 413.18 492.46 Bihar Name of Tiger Reserve Declared in Core Area Buffer Area Total Area Valmiki 1989 598.45 300.93 899.38 Chhattisgarh Name of Tiger Reserve Declared in Core Area Buffer Area Total Area Indravati 1983 1258.37 1540.7 2799.07 Udanti-Sitanadi 2008 851.09 991.45 1842.54 Achanakmar 2008 626.2 287.82 914.02 Jharkhand Name of Tiger Reserve Declared in Core Area Buffer Area Total Area Palamau 1973 414.08 715.85 1129.93 Karnataka Name of Tiger Reserve Declared in Core Area Buffer Area Total Area Bandipur 1973 872.24 584.06 1456.3 Dandeli-Anshi (Kali) 2008 814.88 282.63 1097.51 Bhadra 1998 492.46 571.83 1064.29 Biligiri Ranganatha Temple 2010 359.1 215.72 574.82 Nagarahole 1999 643.35 562.41 1205.76 Kerala Name of Tiger Reserve Declared in Core Area Buffer Area Total Area Parambikulam 2010 390.89 252.77 643.66 Periyar 1978 881 44 925 Madhya Pradesh Name of Tiger Reserve Declared in Core Area Buffer Area Total Area -

DISTRICT SURVEY REPORT for SAND MINING INCLUDING OTHER MINOR MINERAL CHANDRAPUR DISTRICT, MAHARASHTRA
DISTRICT SURVEY REPORT For SAND MINING INCLUDING OTHER MINOR MINERAL CHANDRAPUR DISTRICT, MAHARASHTRA As per Notification No. S.O. 3611 (E) New Delhi, the 25th July, 2018 of Ministry of Environment Forest and Climate change, Government of India Prepared by: District Mining Officer Collector Office, Chandrapur 2019 - 2020 .. ;:- CERTIFICATE The District Survey Report preparation has been undertaken in compliance as per Notification No. S.O. 3611 (E) New Delhi, the 25th July, 2018 of Ministry of Environment Forests and Climate Change, Government of India. Every effort have been made to cover sand mining location, area and overview of mining activity in the district with all its relevant features pertaining to geology and mineral wealth in replenishable and non-replenishable areas of rivers, stream and other sand sources. This report will be a model and guiding document which is a compendium of available mineral resources, geographical set up, environmental and ecological set up of the district and is based on data of various departments, published reports, and websites. The District Survey Report will form the basis for application for environmental clearance, preparation of reports and appraisal of projects. Prepared by: Approved by: ~ District Collector, Chandrapur PREFACE The Ministry of Environment, Forests & Climate Change (MoEF&CC), Government of India, made Environmental Clearance (EC) for mining of minerals mandatory through its Notification of 27th January, 1994 under the provisions of Environment Protection Act, 1986. Keeping in view the experience gained in environmental clearance process over a period of one decade, the MoEF&CC came out with Environmental Impact Notification, SO 1533 (E), dated 14th September 2006. -

Economic Valuation of Tiger Reserves in India a Value+ Approach
ECONOMIC VALUATION OF TIGER RESERVES IN INDIA A VALUE+ APPROACH JANUARY 2015 Centre for Ecological Services Management (CESM), Indian Institute of Forest Management, Bhopal ECONOMIC VALUATION OF TIGER RESERVES IN INDIA A VALUE+ APPROACH JANUARY 2015 Centre for Ecological Services Management (CESM), Indian Institute of Forest Management, Bhopal Supported by Suggested Citation National Tiger Conservation Authority Verma, M., Negandhi, D., Khanna, C., Edgaonkar, Ministry of Environment, Forest & Climate Change, A., David, A., Kadekodi, G., Costanza, R., Singh, R. Government of India, Economic Valuation of Tiger Reserves in India: A Value+ First Floor, East Tower, NBCC Place, Approach. Indian Institute of Forest Management. Bhopal, Bhishma Pitamah Marg, India. January 2015. New Delhi - 110003, India Design Credits Authors Design, Artwork & Cover: Madhu Verma, Innomedia Creations Pvt. Ltd., Kolkata Professor, Indian Institute of Forest Management, Bhopal [email protected] Dhaval Negandhi, Subject Expert, Indian Institute of Forest Management, Bhopal Disclaimer Chandan Khanna, Subject Expert, Indian Institute of Forest Management, Bhopal The views expressed and any errors herein are entirely those of authors. The views as expressed do not necessarily reflect Advait Edgaonkar, those of and cannot be attributed to the study advisors, Assistant Professor, Indian Institute of Forest Management, contacted individuals, institutions and organizations Bhopal involved. The information contained herein has been Ashish David, obtained from various sources -

Carbon Finance: Solution for Mitigating Human–Wildlife Conflict in and Around Critical Tiger Habitats of India
POLICY BRIEF CARBON FINANCE: SOLUTION FOR MITIGATING HUMAN–WILDLIFE CONFLICT IN AND AROUND CRITICAL TIGER HABITATS OF INDIA Author Yatish Lele Dr J V Sharma Reviewers Dr Rajesh Gopal Dr S P Yadav Sanjay Pathak CARBON FINANCE: SOLUTION FOR MITIGATING HUMAN–WILDLIFE CONFLICT IN AND AROUND CRITICAL TIGER HABITATS OF INDIA i © COPYRIGHT The material in this publication is copyrighted. Content from this discussion paper may be used for non-commercial purposes, provided it is attributed to the source. Enquiries concerning reproduction should be sent to the address: The Energy and Resources Institute, Darbari Seth Block, India Habitat Centre, Lodhi Road, New Delhi – 110 003, India Author Yatish Lele, Associate Fellow, Forestry and Biodiversity Division, TERI Dr J V Sharma, Director, Forestry and Biodiversity Division, TERI Reviewers Dr Rajesh Gopal, Secretary General, Global Tiger Forum Dr S P Yadav, Member Secretary, Central Zoo Authority and Former DIG, National Tiger Conservation Authority (NTCA) Sanjay Pathak, Director, Dudhwa Tiger Reserve, Uttar Pradesh Forest Department Cover Photo Credits Yatish Lele ACKNOWLEDGEMENTS This policy brief is part of the project ‘Conservation of Protected Areas through Carbon Finance: Implementing a Pilot Project for Dudhwa Tiger Reserve’ under Framework Agreement between the Norwegian Ministry of Foreign Affairs (MFA) and The Energy and Resources Institute (TERI), referred to in short as the Norwegian Framework Agreement (NFA). We would like to thank the Norwegian MFA and Uttar Pradesh Forest Department for their support. SUGGESTED FORMAT FOR CITATION Lele, Yatish and Sharma, J V 2019. Carbon Finance: Solution for Mitigating Human–wildlife Conflict in and Around Critical Tiger Habitats of India, TERI Policy Brief. -

Global Tiger Forum Is an Inter-Governmental International
GLOBAL TIGER FORUM IS AN INTER-GOVERNMENTAL INTERNATIONAL BODY FOR CONSERVATION OF THE TIGER IN THE WILD GLOBAL TIGER FORUM NEWS Volume 4 No 10 December 2011 Payment to GLOBAL TIGER FORUM The payment to Global Tiger Forum may be made through an Account Payee Cheque or Demand Draft in US dollar payable to Global Tiger Forum at New Delhi Or Please transfer the fee amount to ABN AMRO NY, Swift Code ABNAUS33 for Credit to 574079107542 A/c Bank of Maharastra, Mumbai, under advice to Bank of Maharastra, Connaught Place, New Delhi, Swift Code MAHBINBBCPN for further credit to FCA - A/c 60001719391 of Global Tiger Forum, New Delhi Cover photo courtsey www.tigersintheforest.com GLOBAL TIGER FORUM GLOBAL TIGER FORUM IS AN INTER-GOVERNMENTAL INTERNATIONAL BODY FOR CONSERVATION OF THE TIGER IN THE WILD GTFNEWS Volume 4 No 10 December 2011 EDITOR : S P Yadav Global Tiger Forum Secretariat D-87, Lower Ground Floor, Amar Colony, Raghunath Mandir Road, Lajat Nagar IV New Delhi 110024 GTFNEWS Contents 1. Note from the Secretary General (05) 2. Workshop of Experts to Develop Criteria and Indicators (06) For Monitoring the Global Tiger Recovery Programme 3. News from Countries (12) Bangladesh Cambodia China India Indonesia Malaysia Myanmar Nepal Thailand Vietnam U.K. U.S.A. 4. News from International Agencies/NGOs (32) INTERPOL International Fund For Animal Welfare (IFAW) TRAFFIC International WWF Wildlife Protection Society of India (WPSI) The Corbett Foundation Wildlife Conservation Nepal (WCN) Wildlife Trust of India (WTI) 5. Of the GTF (42) 6. Tiger Mortality and Seizure of Tiger Body Parts, Statistics from India - July to December 2011 (43) 04 December 2011 GTFNEWS NOTE FROM THE SECRETARY GENERAL In the second half of 2011, the Global Tiger Forum, in collaboration with the Global Tiger Initiative, organized a workshop of Experts to develop criteria and indicators for monitoring of the Global Tiger Recovery Programme. -

Ecological Studies of Some Important Tree Species of Subtropical Humid Forest of Meghalaya
Dr. NRIPEMO ODYUO SCIENTIST – D BSI, ERC, SHILLONG Ph.D. Thesis Ecological studies of some important tree species of subtropical humid forest of Meghalaya Guide Prof. H.N. Pandey Dept. of Botany North Eastern Hill University, Shillong, Meghalaya. 2005 BSI, Hqtr.: Scientist – B, 16 th December 2002 BSI, ERC, Shillong: 16 th April 2003 1. Flora of Mt. Saramati, Nagaland: T.M. Hynniewta & N. Odyuo Final Mss. with 278 species under 191 genera and 91 families. submitted to Director BSI, Kolkata in May 2006. New Genus: Penkimia nagalandensis Phukan & Odyuo. New to India: Cleisostoma duplicilobum (J.J. Sm.) Garay 2. Flora of Dampa Tiger Reserve, Mizoram: B.K. Sinha & N. Odyuo Final Mss. with 530 species under 409 genera and 294 families. submitted to Director BSI, Kolkata in March 2008. New to Mizoram: 5 Ancistrocladus tectorius Epithema carnosum Hyptis capitata Phryma leptostachya Fissistigma bicolor Species Endemic to India recorded from the Tiger Reserve: 1.1.1.Argyreia sikkimensis –––Mizoram & Sikkim 2. Mycetia mukherjiana –––Tripura & Mizoram Book Chapter: OdyuoOdyuo,, N. & B.B.T. Tham. 2008. Dampa Tiger Reserve. In Floristic Diversity of Tiger Reserves of India. BSI, Kolkata. pp. 394 –––415. Botanic Garden of Indian Republic, Noida: March 2008 to March 2010 i. Plant seedlings/ saplings procurement tours for introduction at BGIR. 2749 saplings belonging to 82 species mostly of tree species procured from Bhubaneswar and introduced in the Garden. 125 live plants collected from NE India and introduced in the Garden. ii. Seed Bank Development programme. Seed germination lab. made operational with procurement of basic equipments. Collected seeds of 37 species from NE India, processed and stored Seeds of 63 species of BGIR plants collected, processed & stores iii. -

Wildlife Sanctuaries of India
Wildlife sanctuaries of India Srisailam Sanctuary, Andhra Pradesh The largest of India's Tiger Reserves, the Nagarjunasagar Srisailam Sanctuary ( 3568 sq. km.); spreads over five districts - Nalgonda, Mahaboobnagar, Kurnool, Prakasam and Guntur in the state of Andhra Pradesh. The Nagarjunasagar-Srisailam Sanctuary was notified in 1978 and declared a Tiger Reserve in 1983. The Reserve was renamed as Rajiv Gandhi Wildlife Sanctuary in 1992. The river Krishna flows through the sanctuary over a distance of 130 km. The multipurpose reservoirs, Srisailam and Nagarjunasagar, which are important sources of irrigation and power in the state are located in the sanctuary. The reservoirs and temples of Srisailam are a major tourist and pilgrim attraction for people from all over the country and abroad. The terrain is rugged and winding gorges slice through the Mallamalai hills. Adjoining the reserve is the large reservoir of the Nagarjunasagar Dam on the River Krishna. The dry deciduous forests with scrub and bamboo thickets provide shelter to a range of animals from the tiger and leopard at the top of the food chain, to deer, sloth bear, hyena, jungle cat, palm civet, bonnet macaque and pangolin. In this unspoilt jungle, the tiger is truly nocturnal and is rarely seen. Manjira Wildlife Sanctuary Total Area: 3568-sq-kms Species found: Catla, Rahu, Murrel, Ech Paten, Karugu, Chidwa,Painted Storks, Herons, Coots, Teals, Cormorants, Pochards, Black and White Ibises, Spoon Bills, Open Billed Storks etc About Manjira Wildlife Sanctuary: Manjira bird sanctuary spreads over an area of 20 sq.kms and is the abode of a number of resident and migratory birds and the marsh crocodiles. -
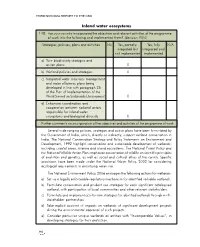
CBD Third National Report
THIRD NATIONAL REPORT TO THE CBD Inland water ecosystems 148. Has your country incorporated the objectives and relevant activities of the programme of work into the following and implemented them? (decision VII/4) Strategies, policies, plans and activities No Yes, partially, Yes, fully N/A integrated but integrated and not implemented implemented a) Your biodiversity strategies and action plans X b) Wetland policies and strategies X c) Integrated water resources management and water efficiency plans being developed in line with paragraph 25 of the Plan of Implementation of the World Summit on Sustainable Development X d) Enhanced coordination and cooperation between national actors responsible for inland water ecosystems and biological diversity X Further comments on incorporation of the objectives and activities of the programme of work Several wide ranging policies, strategies and action plans have been formulated by the Government of India, which, directly or indirectly, support wetland conservation in India. The National Conservation Strategy and Policy Statements on Environment and Development, 1992 highlight conservation and sustainable development of wetlands, including coastal areas, riverine and island ecosystems. The National Forest Policy and the National Wildlife Action Plan emphasize conservation of wildlife on scientific principles of evolution and genetics, as well as social and cultural ethos of the county. Specific provisions have been made under the National Water Policy, 2002 for considering ecological requirements in prioritizing water use. The National Environment Policy, 2006 envisages the following actions for wetlands: a) Set up a legally enforceable regulatory mechanism for identified valuable wetlands. b) Formulate conservation and prudent use strategies for each significant catalogued wetland, with participation of local communities and other relevant stakeholders. -

Importance of Tiger Conservation
Importance of Tiger Conservation drishtiias.com/printpdf/importance-of-tiger-conservation Watch Video At: https://youtu.be/ck91LsuizJU The tiger population across the world dropped sharply since the beginning of the 20 th century but now for the first time in conservation history, their numbers are on the rise. India has 70% of the global tiger population. The International Tiger Day celebrated on 29th july is an annual event marked to raise awareness about tiger conservation. First international Tiger’s day was celebrated in 2010 at the St. Petersburg Tiger Summit. St. Petersburg Declaration on Tiger Conservation 1/5 This resolution was adopted In November 2010, by the leaders of 13 tiger range countries (TRCs) assembled at an International Tiger Forum in St. Petersburg, Russia It aimed at promoting a global system to protect the natural habitat of tigers and raise awareness among people on white tiger conservation. The resolution’s implementation mechanism is called the Global Tiger Recovery Program whose overarching goal was to double the number of wild tigers from about 3,200 to more than 7,000 by 2022. 13 Tiger range countries are: Bangladesh, Bhutan, Cambodia, China, India, Indonesia, Laos, Malaysia, Myanmar, Nepal, Russia, Thailand and Vietnam. Encouraging Factors in Conservation of Tigers According to the Tiger Census Report, 2019, the Tiger population has substantially increased from 2,226 in 2014 to around 2,967 in 2019. Key Findings Top Performers: Madhya Pradesh saw the highest number of tigers (526) followed by Karnataka (524) and Uttarakhand (442). Increase in Tiger population: Madhya Pradesh (71%) > Maharashtra (64%) > Karnataka (29%). -

District Survey Report for Sand Mining Or River Bed Mining
Draft DSR Report for Gondia DISTRICT SURVEY REPORT FOR SAND MINING OR RIVER BED MINING Prepared by District Mining Officer Collector Office, Gondia Prepared Under A] Appendix –X Of MoEFCC, GoI. Notification S.O. 141(E) Dated 15.1.2016 B] Sustainable Sand Mining Guidelines C] MoEFCC, GoI. Notification S.O. 3611(E) Dated 25.07.2018 DISTRICT-GONDIA MAHARASHTRA PREFACE With reference to the gazette notification dated 15th January 2016, ministry of Environment, Forest and Climate Change, the State environment Impact Assessment Authority (SEIAA) and State Environment Assessment Committee (SEAC) are to be constituted by the divisional commissioner for prior environmental clearance of quarry for minor minerals. The SEIAA and SEAC will scrutinize and recommend the prior environmental clearance of ministry of minor minerals on the basis of district survey report. The main purpose of preparation of District Survey Report is to identify the mineral resources and mining activities along with other relevant data of district. This report contains details of Lease, Sand mining and Revenue which comes from minerals in the district. This report is prepared on the basis of data collected from different concern departments. A survey is carried out by the members of DEIAA with the assistance of Geology Department or Irrigation Department or Forest Department or Public Works Department or Ground Water Boards or Remote Sensing Department or Mining Department etc. in the district. Minerals are classified into two groups, namely (i) Major minerals and (ii) Minor minerals. Amongst these two groups minor mineral have been defined under section 3(e) of Mines and Minerals (Regulation and development) Act, 1957.