Pigments and Paints
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

HISTORY of LEAD POISONING in the WORLD Dr. Herbert L. Needleman Introduction the Center for Disease Control Classified the Cause
HISTORY OF LEAD POISONING IN THE WORLD Dr. Herbert L. Needleman Introduction The Center for Disease Control classified the causes of disease and death as follows: 50 % due to unhealthy life styles 25 % due to environment 25% due to innate biology and 25% due to inadequate health care. Lead poisoning is an environmental disease, but it is also a disease of life style. Lead is one of the best-studied toxic substances, and as a result we know more about the adverse health effects of lead than virtually any other chemical. The health problems caused by lead have been well documented over a wide range of exposures on every continent. The advancements in technology have made it possible to research lead exposure down to very low levels approaching the limits of detection. We clearly know how it gets into the body and the harm it causes once it is ingested, and most importantly, how to prevent it! Using advanced technology, we can trace the evolution of lead into our environment and discover the health damage resulting from its exposure. Early History Lead is a normal constituent of the earth’s crust, with trace amounts found naturally in soil, plants, and water. If left undisturbed, lead is practically immobile. However, once mined and transformed into man-made products, which are dispersed throughout the environment, lead becomes highly toxic. Solely as a result of man’s actions, lead has become the most widely scattered toxic metal in the world. Unfortunately for people, lead has a long environmental persistence and never looses its toxic potential, if ingested. -
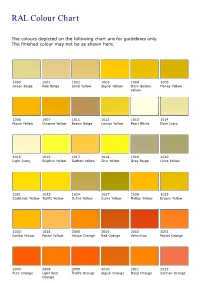
RAL Colour Chart
RAL Colour Chart The colours depicted on the following chart are for guidelines only. The finished colour may not be as shown here. 1000 1001 1002 1003 1004 1005 Green Beige Pale Beige Sand Yellow Signal Yellow Dark Golden Honey Yellow Yellow 1006 1007 1011 1012 1013 1014 Maize Yellow Chrome Yellow Brown Beige Lemon Yellow Pearl White Dark Ivory 1015 1016 1017 1018 1019 1020 Light Ivory Sulphur Yellow Saffron Yellow Zinc Yellow Grey Beige Olive Yellow 1021 1023 1024 1027 1028 1032 Cadmium Yellow Traffic Yellow Ochre Yellow Curry Yellow Mellon Yellow Broom Yellow 1033 1034 2000 2001 2002 2003 Dahlia Yellow Pastel Yellow Yellow Orange Red Orange Vermillion Pastel Orange 2004 2008 2009 2010 2011 2012 Pure Orange Light Red Traffic Orange Signal Orange Deep Orange Salmon Orange Orange 3000 3001 3002 3003 3004 3005 Flame Red RAL Signal Red Carmine Red Ruby Red Purple Red Wine Red 3007 3009 3011 3012 3013 3014 Black Red Oxide Red Brown Red Beige Red Tomato Red Antique Pink 3015 3016 3017 3018 3020 3022 Light Pink Coral Red Rose Strawberry Red Traffic Red Dark Salmon Red 3027 3031 4001 4002 4003 4004 Raspberry Red Orient Red Red Lilac Red Violet Heather Violet Claret Violet 4005 4006 4007 4008 4009 4010 Blue Lilac Traffic Purple Purple Violet Signal Violet Pastel Violet Telemagenta 5000 5001 5002 5003 5004 5005 Violet Blue Green Blue Ultramarine Blue dark Sapphire Black Blue Signal Blue Blue 5007 5008 5009 5010 5011 5012 Brilliant Blue Grey Blue Light Azure Blue Gentian Blue Steel Blue Light Blue 5013 5014 5015 5017 5018 5019 Dark Cobalt Blue -

Watercolours: What´S Different in the New Assortment?
Product information Watercolours HORADAM® watercolours: What´s different in the new assortment? The following colours have got a new name: Art.-No. Old Name New Name 14 208 Aureolin modern Aureolin hue 14 209 Translucent yellow Transparent Yellow 14 211 Chrome yellow lemon Chromium yellow hue lemon 14 212 Chrome yellow light Chromium yellow hue light 14 213 Chrome yellow deep Chromium yellow hue deep 14 214 Chrome orange Chromium orange hue 14 218 Translucent orange Transparent orange 14 366 Deep red Perylene maroon 14 476 Mauve Schmincke violet 14 498 Dark blue indigo Dark blue 14 648 Sepia brown tone Sepia brown reddish 14 786 Charcoal grey Anthracite The following colours are completely omitted due to raw materials which aren’t available anymore: Art.-No. Old Name 14 652 Walnut brown This tone cannot be mixed out of other colours. 14 666 Pozzuoli earth This tone can be mixed using 14 649 English Venetian red and the slightly reddish 14 670 Madder brown. These colours are now produced using other pigments. Therefore they have got a new name and a new number: Old Art.-No. Old Name New Art.-No. New Name 14 345 Dark red 14 344 Perylene dark red 14 210 Gamboge gum modern 14 217 Quinacridone gold hue 14 478 Helio blue reddish 14 477 Phthalo sapphire blue 14 536 Green yellow 14 537 Transparent green gold - Discontinued colours The described product attributes and application examples have been tes- a warranty for product attributes and/or assume liability for damages that ted in the Schmincke laboratory. -

RAL COLOR CHART ***** This Chart Is to Be Used As a Guide Only. Colors May Appear Slightly Different ***** Green Beige Purple V
RAL COLOR CHART ***** This Chart is to be used as a guide only. Colors May Appear Slightly Different ***** RAL 1000 Green Beige RAL 4007 Purple Violet RAL 7008 Khaki Grey RAL 4008 RAL 7009 RAL 1001 Beige Signal Violet Green Grey Tarpaulin RAL 1002 Sand Yellow RAL 4009 Pastel Violet RAL 7010 Grey RAL 1003 Signal Yellow RAL 5000 Violet Blue RAL 7011 Iron Grey RAL 1004 Golden Yellow RAL 5001 Green Blue RAL 7012 Basalt Grey Ultramarine RAL 1005 Honey Yellow RAL 5002 RAL 7013 Brown Grey Blue RAL 1006 Maize Yellow RAL 5003 Saphire Blue RAL 7015 Slate Grey Anthracite RAL 1007 Chrome Yellow RAL 5004 Black Blue RAL 7016 Grey RAL 1011 Brown Beige RAL 5005 Signal Blue RAL 7021 Black Grey RAL 1012 Lemon Yellow RAL 5007 Brillant Blue RAL 7022 Umbra Grey Concrete RAL 1013 Oyster White RAL 5008 Grey Blue RAL 7023 Grey Graphite RAL 1014 Ivory RAL 5009 Azure Blue RAL 7024 Grey Granite RAL 1015 Light Ivory RAL 5010 Gentian Blue RAL 7026 Grey RAL 1016 Sulfer Yellow RAL 5011 Steel Blue RAL 7030 Stone Grey RAL 1017 Saffron Yellow RAL 5012 Light Blue RAL 7031 Blue Grey RAL 1018 Zinc Yellow RAL 5013 Cobolt Blue RAL 7032 Pebble Grey Cement RAL 1019 Grey Beige RAL 5014 Pigieon Blue RAL 7033 Grey RAL 1020 Olive Yellow RAL 5015 Sky Blue RAL 7034 Yellow Grey RAL 1021 Rape Yellow RAL 5017 Traffic Blue RAL 7035 Light Grey Platinum RAL 1023 Traffic Yellow RAL 5018 Turquiose Blue RAL 7036 Grey RAL 1024 Ochre Yellow RAL 5019 Capri Blue RAL 7037 Dusty Grey RAL 1027 Curry RAL 5020 Ocean Blue RAL 7038 Agate Grey RAL 1028 Melon Yellow RAL 5021 Water Blue RAL 7039 Quartz Grey -

Standard Operating Procedure for the Preparation of Lead-Containing Paint Films and Lead-In-Paint Diagnostic Test Materials
EPA/600/R-10/070 August 2009 www.epa.gov/ord Standard Operating Procedure for the Preparation of Lead-Containing Paint Films and Lead-in-Paint Diagnostic Test Materials Prepared by Kristen Sorrell, David Binstock, Curtis Haas, Cynthia Salmons, and William Gutknecht Environmental and Industrial Sciences Division RTI International Research Triangle Park, NC 27709 Disclaimer The information in this document has been funded wholly or in part by the U. S. Environmental Protection Agency (EPA) under EPA Contract No. EP-D-05-065 to Alion Science and Technology, Inc., and RTI Subcontract No. SUB1174861RB. It has been subjected to the Agency’s peer and administrative review. Mention of trade names or commercial products does not constitute endorsement or recommendation for use. ii Acknowledgments This document was prepared under the direction of the Work Assignment Contracting Officer’s Representative, Ms. Sharon L. Harper, National Environmental Research Laboratory, U.S. Environmental Protection Agency, Research Triangle Park, NC. Special acknowledgment is given to Dr. Hunter Daughtrey, Alion Science and Technology, Inc., for his support of this effort and careful review of this document. iii iv Table of Contents List of Figures...............................................................................................................................................vii List of Appendixes........................................................................................................................................vii 1.0 PRINCIPLE AND -

A Short History of 18-19Th Century
A SHORT HISTORY OF 18-19TH CENTURY BRITISH HAND-COLOURED PRINTS; WITH A FOCUS ON GAMBOGE, CHROME YELLOW AND QUERCITRON; THEIR SENSITIVITIES AND THEIR IMPACT ON AQUEOUS CONSERVATION TREATMENTS Stacey Mei Kelly (13030862) A Dissertation presented at Northumbria University for the degree of MA in Conservation of Fine Art, 2015 VA0742 Page 1 of 72 Table of Contents List of figures……………………………………………………………………………………...…2 List of tables……………………………………………………………………………………….....2 Abstract………………………………………………………………………………………………3 Introduction……………………………………………………………………………..………...…3 Research aims, methodology and resources………………………………………………….…....4 1. Aims……………………………………………………………………………………..…...4 2. Research Questions…………………………………………………………………..…...….4 3. Literature review……………………………………………………………………….....….5 4. Case Study Survey………………………………………………………………………..….5 5. Empirical Work……………………………………………………………………………....6 Chapter 1: A Brief History of Hand-coloured Prints in Britain………………………………....6 1.1 The popularity of hand-coloured prints…………………………………………………….....6 1.2 The people behind hand-colouring……………………………………………………..….….9 1.3 Materials and Methods……………………………………………………………………....14 Chapter 2: Yellow Pigments: A focus on Gamboge, Chrome Yellow, and Quercitron……….19 2.1 Why Gamboge, Chrome Yellow, and Quercitron……………………………………….…..19 2.2 Gamboge……………………………………………………………………………….....….20 2.2.1 History…………………………………………………………………………….…....20 2.2.2 Working properties…………………………………………………………………..…21 2.2.3 Physical and chemical properties…………………………………………………..…..21 2.2.4 Methods of -

Toxicological Profile for Lead
TOXICOLOGICAL PROFILE FOR LEAD U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Agency for Toxic Substances and Disease Registry August 2007 LEAD ii DISCLAIMER The use of company or product name(s) is for identification only and does not imply endorsement by the Agency for Toxic Substances and Disease Registry. LEAD iii UPDATE STATEMENT A Toxicological Profile for Lead, Draft for Public Comment was released in September 2005. This edition supersedes any previously released draft or final profile. Toxicological profiles are revised and republished as necessary. For information regarding the update status of previously released profiles, contact ATSDR at: Agency for Toxic Substances and Disease Registry Division of Toxicology and Environmental Medicine/Applied Toxicology Branch 1600 Clifton Road NE Mailstop F-32 Atlanta, Georgia 30333 LEAD iv This page is intentionally blank. v FOREWORD This toxicological profile is prepared in accordance with guidelines developed by the Agency for Toxic Substances and Disease Registry (ATSDR) and the Environmental Protection Agency (EPA). The original guidelines were published in the Federal Register on April 17, 1987. Each profile will be revised and republished as necessary. The ATSDR toxicological profile succinctly characterizes the toxicologic and adverse health effects information for the hazardous substance described therein. Each peer-reviewed profile identifies and reviews the key literature that describes a hazardous substance's toxicologic properties. Other pertinent literature is also presented, but is described in less detail than the key studies. The profile is not intended to be an exhaustive document; however, more comprehensive sources of specialty information are referenced. The focus of the profiles is on health and toxicologic information; therefore, each toxicological profile begins with a public health statement that describes, in nontechnical language, a substance's relevant toxicological properties. -

Examination of Pigments on Thai Manuscripts: the first Identification of Copper Citrate
JOURNAL OF RAMAN SPECTROSCOPY J. Raman Spectrosc. 2008; 39: 1057–1065 Published online 27 May 2008 in Wiley InterScience (www.interscience.wiley.com) DOI: 10.1002/jrs.1985 Examination of pigments on Thai manuscripts: the first identification of copper citrate Katherine Eremin,1∗ Jens Stenger,1 Jo-Fan Huang,3 Alan Aspuru-Guzik,2 Theodore Betley,2 Leslie Vogt,2 Ivan Kassal,2 Scott Speakman4 and Narayan Khandekar1 1 Harvard University Art Museums, 32 Quincy Street, Cambridge, MA 02138, USA 2 Department of Chemistry and Chemical Biology, 12 Oxford Street, Harvard University, Cambridge, MA 02138, USA 3 Philadelphia Museum of Art, Benjamin Franklin Parkway, Philadelphia, PA 19130, USA 4 Center for Materials Science and Engineering, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139, USA Received 13 December 2007; Accepted 5 March 2008 Samples from Thai manuscripts dated to the 18th to 20th century were analyzed by Raman spectroscopy and Fourier-transform infrared spectroscopy (FTIR) to determine the pigments used. This suggested a change in palette from the 18th to 20th century, with use of imported pigments in the later manuscripts. In the 18th century, the main green used was an organic copper salt, which was replaced by emerald green and mixtures of Prussian blue with gamboge, chrome yellow and zinc yellow (zinc potassium chromate). Chrome yellow was used in addition to gamboge in one later 19th century manuscript. Similarly, indigo in the 18th century manuscripts was replaced by Prussian blue and then synthetic ultramarine in the 19th century manuscripts. Lead white was the main white pigment in all but one manuscript, which contained huntite, a magnesium calcium carbonate. -

Paint Pigments— Yellow
» TECHNICAL INFORMATION ON BUILDING MATERIALS TIBM - 32 FOR UfSE IN THE DESIGN OF LOW-COST HOUSING ***** THE NATIONAL BUREAU OF STANDARDS UNITED STATES DEPARTMENT OF COMMERCE WASHINGTON, D. C. August 29, 1936 PAINT PIGMENTS— YELLOW, . BROWN, BLUE, GREEN, AND BRONZE This is urimarily^a digest of the sections of Bureau of Standards Circular No, o9> "Paint and Varnish", (November 17, 1917),'*' and Tech- nologic Paper No. 274, "Use of United States Government Suecif ication Paints and Faint Materials", (December 15, 1924), ^ Ly p, H. Walker and E. F. Hickson, dealing with general composition , characteristics, and uses of yellow, brown, blue, green, and bronze pigments. The following papers contain additional information relative to paint pigments, oil paints, and water paints: TIBM - 30 "Paint Pigments—White" TIBM - 31 "Paint Pigments—Black, Red, and Lakes" TIBM - 33 "Federal Specification . Paint Pigments and Mixing Formulas" TIM - 3U "Federal Specification Ready-Mixed Paints, Semi- paste Paints and Mixing Formulas’"' TIBM - 35 "Preparation of Paints from Paste and Dry Pigments" TIBM - 36 "Preparation of Paints from Semipaste Paints, Thinning Ready-Mixed Paints, and Preparation of Water Paints" TIBM - 43 "Aluminum Paints" Pigments are "the fine solid warticles used in the preparation of paint, and substantially insoluble in the vehicle, "3 In general, it may be ^Out of print. May be consulted in Government depositor}*- libraries. p Available from Superintendent of Documents, Government Printing Office, Washington, D. C. .(Price 10 cents). ^Qpioted from "Standard Definitions of Terms Relating to Paint 'Specifications", American Society for Testing Materials ( 1 93 3 ) ’ • • -• •• PP. 735-73 9 . 031736-C - 1 - assumed that pigments composed of very fine particles, having high re- fractive indices, provide the greatest covering power and opacity. -

EF MAXOPAKE CHROME YELLOW Product Overview Product Code PADE2020 Instructions Industry Inks Dark Garments
Recommendations EF MAXOPAKE CHROME YELLOW Product Overview Product Code PADE2020 Instructions Industry Inks Dark garments. Direct printing. 100% cotton or cotton/polyester.* Stencils: Use any direct emulsion or Application Screen Printing capillary film compatible with plastisol inks. Additives: Maxopake inks are supplied ready to print. If necessary to reduce viscosity, use up to 15% by weight, of Reducer/Detackifi er (PLRE-9000). For printing Category Stock Colors transfers, mix Maxopake with with 5-10% Hot Split Additive (PLUE-9040). Printing Instructions: Multiple Chemistry Plastisol strokes may be required when printing by hand. When printing with automatic presses use a slightly rounded squeegee to print a thicker ink layer. A soft pad on the printing pallet will minimize penetration Substrate(s) Cotton into the garment and improve opacity. Curing Instructions: These inks will fully cure when the entire Best Used By 12 months thickness of the ink deposit reaches 300°•F (149°•C). Using Low-Bleed Inks: The Maxopake series includes two low-bleed colors: Low-Bleed Medium Yellow (PADE-2060), and Low-Bleed Golden Yellow Certification(s) ISO9001 (PADE-2048). The lowbleed inks are recommended for printing on cotton/polyester garments to control Performance: the problem of dyes in the polyester fi bers migrating or _ã–bleeding_ã• into the plastisol ink. Low-bleed inks are not recommended for printing on light-colored 100% cotton fabrics. On rare occasions ghost After Flash Tack Decreases with increased images can appear on contacting surfaces of ink to garment. The use of low-bleed plastisols on these mesh fabrics is not recommended. If low-bleed colors are used, be sure to fully cure, to minimize the possibility Squeegee: of ghost images. -

Aug. 25, 1964 R, G, JELLY Plant Pat. 2,443 ROSE PLANT Filed Oct
Aug. 25, 1964 R, G, JELLY Plant Pat. 2,443 ROSE PLANT Filed Oct. 18, 1963 Plant Pat. 2,443 United States Patent 0 "cc Patented Aug. 25, 1964 1 2 2,443 Pollen parent.—An unnamed seedling identi?ed as RUSE PLANT #l-57-R. Robert G. Jelly, Richmond, Ind, assignor to E. G. Hill Propagation: Holds its distinguishing characteristics Co., Inc, Richmond, End, a corporatien of llndiana through succeeding propagations by grafting. Filed Oct. 15, 1963, Ser. No. 317,394 Flower 1 Claim. (Cl. Plt.—2tl) Locality where grown and observed: Richmond, Indiana. 1 The present invention relates to a new and distinct Flowers borne: One to a stern; on strong stems of from variety of rose plant of the hybrid tea class, which was medium to long length. 7 originated by me by crossing the variety “Yuletide” (Plant 10 Quantity of bloom: Abundant, in greenhouse. Patent No. 1,391) with an unnamed and unpatented Continuity: Continuous, in greenhouse. seedling identi?ed in my breeding records as #l-57-R. Fragrance: Penetrating, in greenhouse. Nature—spicy. ' The primary objective of this breeding was to produce Bud: a new rose variety in which there is combined the vigor Pedzmcle.-Medium length; from large to medium and growth habits of the unnamed pollen parent, and 15 diameter; erect; strong. Bark—slightly rough, the keeping quality and flower color of the seed parent due to numerous minute prickles of Sulphur Yel “Yuletide.” This objective was fully achieved along with low color, Plate 1/3 (W); no thorns or hairs. other desirable improvements‘, as evidenced by the follow Color—Moderate Yellow Green, Plate SGY 5/6 ing unique combination of characteristics which are out (N), shaded to'Strong Yellow Green, Plate 5GY standing in the new variety and which distinguish it from 20 6/8 (N) at base of bud. -

A STANDARD of QUALITY for MUSE PAINT January 1949
Ät1001:31TUP,E ROOT 5 A STANDARD Of QUALITY FOR MUSE PAINT January 1949 No. 1)1734 UNITED STATES DEPARTMENT OF AGRICULTURE FOREST SERVICE FORE ST PRODUCTS LABORATORY Madison 5, Wisconsin In Cooperation with the University of Wisconsin A STANDARD OF QUALITY FOR HOUSE PAINT By F. L. BROWNE, Chemist 1 Forest Products Laboratory, – Forest Service U. S. Department of Agriculture You can now buy house paint and barn paints labeled by the maker with both his trade brand and a classification recommended by the U. S. Depart- ment of Agriculture. The classification is a form of quality standard. It tells briefly what kind and grade of paint you are buying. The trade brand tells, as it always has, who made the paint and expects to be held respon- sible for it. Together, classification and trade brand supply all that is needed to buy from reputable sources with an eye to quality as well as to price. The USDA classification plan was suggested by the Forest Products Laboratory after more than 20 years of research in the painting of wood. Under the plan there is a classification for every kind of house or barn paint for wood buildings. Any maker or dealer in paint is free to use the classification on his labels. No law requires it, however, and no agency of government need be consulted about it. But when the classification is used, the paint must conform in fact to the classification claimed for it. Mis- branded paint is unlawful. The law in some States requires the composition, or formula, of paint to be printed on the label.