Experimental Organic Chemistry
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Docteur» at the University François Rabela
UNIVERSITÉ FRANÇOIS – RABELAIS DE TOURS École Doctorale « Santé - Sciences Biologiques - Chimie du Vivant » and UNIVERSITY OF LJUBLJANA, FACULTY OF PHARMACY «Department of Pharmaceutical Chemistry» A cotutelle thesis submitted in fulfillment of the requirements for the degree of «Docteur» at the University François Rabelais of Tours (France) and Doctor of Pharmacy at the University of Ljubljana (Slovenia) In Pharmaceutical Chemistry Publicly defended on the 1st of March 2013 by Mitja KOVAČ in Ljubljana FLUORATION DE DERIVES DU BENZOVESAMICOL POUR L'OBTENTION DE RADIOLIGANDS POTENTIELS DU TRANSPORTEUR VESICULAIRE DE L'ACETYLCHOLINE Under the co-direction of: Associate Professor Sylvie Mavel (MCU, Tours) and Associate Professor Marko Anderluh (Ljubljana) ----------------- JURY for Oral Defense: Ms MAVEL Sylvie – Associate Professor, University François-Rabelais, Tours, France Mr ANDERLUH Marko – Associate Professor, University of Ljubljana, Slovenia Mr DOLLÉ Frédéric – Service Hospitalier Frédéric Joliot, Institut d'Imagerie BioMédicale - CEA, Orsay, France (Reviewer) Mr EMOND Patrick – Professor, University François-Rabelais, Tours, France Ms GMEINER STOPAR Tanja – Assistant Professor, University of Ljubljana, Slovenia (Reviewer) Mr GOBEC Stanislav – Professor, University of Ljubljana, Slovenia (Chairman) This cotutelle PhD was carried out with the collaboration between the University of Tours (Laboratoire de Biophysique Médicale et Pharmaceutique, Unité INSERM U930 - FRANCE) and the University of Ljubljana (Faculty of Pharmacy, Department of Pharmacutical Chemistry - SLOVENIA). The work was supported by a grant from the Slovene Human Resources Development and Scholarship Fund, by a grant from the University of Ljubljana (Inovativna shema za sofinanciranje doktorskega študija za spodbujanje sodelovanja z gospodarstvom in reševanja aktualnih družbenih izzivov - generacija 2010 Univerza v Ljubljani), and by a Slovenia- French bilateral collaboration project (project n° BI-FR/12-13-PROTEUS-007). -

The Ozonolysis of Phenyl Grignard Reagent
University of Montana ScholarWorks at University of Montana Graduate Student Theses, Dissertations, & Professional Papers Graduate School 1971 The ozonolysis of phenyl Grignard reagent Gale Manning Sherrodd The University of Montana Follow this and additional works at: https://scholarworks.umt.edu/etd Let us know how access to this document benefits ou.y Recommended Citation Sherrodd, Gale Manning, "The ozonolysis of phenyl Grignard reagent" (1971). Graduate Student Theses, Dissertations, & Professional Papers. 8297. https://scholarworks.umt.edu/etd/8297 This Thesis is brought to you for free and open access by the Graduate School at ScholarWorks at University of Montana. It has been accepted for inclusion in Graduate Student Theses, Dissertations, & Professional Papers by an authorized administrator of ScholarWorks at University of Montana. For more information, please contact [email protected]. THE OZONOLYSIS OF PHENYL GRIGNARD REAGENT By Gale M. Sherrodd B.S., Rocky Mountain College, I969 Presented in partial fulfillment of the requirements for the degree of Master of Arts for Teachers UNIVERSITY OF MONTANA 1971 Approved by: Chairman, Board of Examiners De^ , Graduate *School / n ? / Date Reproduced with permission of the copyright owner. Further reproduction prohibited without permission. UMI Number: EP39098 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. UMT DiMMtstion PuWiahing UMI EP39098 Published by ProQuest LLC (2013). Copyright in the Dissertation held by the Author. -

TOXICOLOGICAL REVIEW of BROMOBENZENE (CAS No
DRAFT - DO NOT CITE OR QUOTE EPA/635/R-07/002 www.epa.gov/iris TOXICOLOGICAL REVIEW OF BROMOBENZENE (CAS No. 108-86-1) In Support of Summary Information on the Integrated Risk Information System (IRIS) June 2007 NOTICE This document is an interagency review draft. It has not been formally released by the U.S. Environmental Protection Agency and should not at this stage be construed to represent Agency position on this chemical. It is being circulated for review of its technical accuracy and science policy implications. U.S. Environmental Protection Agency Washington, DC DISCLAIMER This document is a preliminary draft for review purposes only and does not constitute U.S. Environmental Protection Agency policy. Mention of trade names or commercial products does not constitute endorsement or recommendation for use. 6/7/07 ii DRAFT – DO NOT CITE OR QUOTE CONTENTSCTOXICOLOGICAL REVIEW OF BROMOBENZENE (CAS No. 108-86-1) LIST OF FIGURES ....................................................................................................................... vi LIST OF TABLES........................................................................................................................ vii LIST OF ABBREVIATIONS AND ACRONYMS ........................................................................x FOREWORD ................................................................................................................................. xi AUTHORS, CONTRIBUTORS, AND REVIEWERS ................................................................ xii -

Reactivity of Nucleophiles Toward Phenyl Radical James Louis Anderson Jr
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1986 Reactivity of nucleophiles toward phenyl radical James Louis Anderson Jr. Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Organic Chemistry Commons Recommended Citation Anderson, James Louis Jr., "Reactivity of nucleophiles toward phenyl radical " (1986). Retrospective Theses and Dissertations. 8135. https://lib.dr.iastate.edu/rtd/8135 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS While the most advanced technology has been used to photograph and reproduce this manuscript, the quality of the reproduction is heavily dependent apon the quality of the material submitted. For example: • Manuscript pages may have indistinct print. In such cases, the best available copy has been filmed. • Manuscripts may not always be complete. In such cases, a note will indicate that it is not possible to obtain missing pages. • Copyrighted material may have been removed from the manuscript. In such cases, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, and charts) are photographed by sectioning the original, beginning at the upper left-hand comer and continuing fiijm left to right in equal sections with small overlaps. Each oversize page is also filmed as one exposure and is available, for an additional charge, as a standard 35mm slide or as a 17"x 23" black and white photographic print. -

Friction of Iron Lubricated with Aliphatic and Aromatic Hydrocarbons and Halogenated Analogs
NASA TECHNICAL NOTE 00 0 N w n a c 4 &A 4 a w~.~,z..p~COPY: RETURN TO --;F?.f-..wL TECHNICAL LIBRARY 1(LWTWND ATS, M* M* FRICTION OF IRON LUBRICATED WITH ALIPHATIC AND AROMATIC HYDROCARBONS AND HALOGENATED ANALOGS Donald H. Buckley Lewis Research Center NATIONAL AERONAUTICS AND SPACE ADMINISTRATION WASHINGTON, D. C. APRIL 1976 TECH LIBRARY KAFB,"I .-- - -.- ~~ OL337b7 I 1. Report No. I 2. Government Accession No. ]Recipient's Catalog NO. TN D -8208 I- .- .. I I 4. Title and Subtitle 5. Report Date FRICTION OF IRON LUBRICATED WITH ALIPHATIC AND I April 1976 Performing OrganizationCode AROMATIC HYDROCARBONS AND HALOGENATED ANALOGS I 7. Author(s1 8. Performing Organization Report No. E-8558 Donald H. Buckley ~__ .. 10. Wcrk Unit No. 9. Performing Organization Name and Address 506-16 Lewis Research Center 11. Contract or Grant No. National Aeronautics and Space Administration I Cleveland, Ohio 44135 13. Type of Report and Period Covered ~~ 12. Sponsoring Agency Name and Address Technical Note National Aeronautics and Space Administration 14. Sponsoring Agency Code Washington, D.C. 20546 I 1 15. Supplementary Notes - -- L16. Abstract An investigation was conducted to determine the influence of oxygen and various organic mole cules on the reduction of the friction of an iron (011) single crystal surface. A comparison was made between aliphatic and aromatic structures, all of which contained six carbon atoms, and among various halogen atoms. Results of the investigation indicate that hexane and benzene give similar friction coefficients over a range of loads except at very light loads. At light loads, the friction decreased with an increase in the load where the halogens fluorine and chlorine are in corporated into the benzene molecular structure; however, over the same load range when bro mine and iodine were present, the friction was relatively unchanged. -

UNITED STATES PATENT OFFICE 2,677,686 PYRAZINEDERVATIVES and METHO) of PREPARING the SAME Victor K
Patented May 4, 1954 2,677,686 UNITED STATES PATENT OFFICE 2,677,686 PYRAZINEDERVATIVES AND METHO) OF PREPARING THE SAME Victor K. Smith, Jr., Feari River, and Samuel Kushner, Nanuet, N. Y., assignors to American Cyanamid Company, New York, N. Y., a corpo ration of Maine No Drawing. Application 5uly 22, 1952, Serial No. 300,336 8 Claims. (C. 260-250) 2 This invention relates to mono-substituted ous layer is extracted with a solvent, such as di pyrazine. More particularly, it relates to substi ethyl ether. The ether can be concentrated and tuted-2-carbonyl pyrazine. the desired compound crystallized out Or the The role of vitamins in nutrition is well known ether can be removed and the product distilled. i and assumes greater importance as new informa 5, The reaction of the present invention may be tion is made available concerning the particular carried out at a temperature of -10° to 15° C. function of each. Recently it has been found The reaction is complete in from about a few that folic acid was effective in curing macrocytic ininutes up to about tWO hours. anemias and other blood conditions. Also, Com The process of the present invention is de pounds which are folic acid antagonistS, Such as scribed in greater particularity by the following aninopterin, have been found useful in treating specific examples which are given by way of ill abnormal blood conditions such as leukemia. lustration and not limitation. it is well established that nicotinamide is an im Eacample 1 portant, vitamin of the B complex group and its deficiency is the specific cause of pelagira. -

United Sttes Atent 0 ICC Patented Feb
p , ‘ 2,924,620 United Sttes atent 0 ICC Patented Feb. 9, 1960 1 2 gish or fails entirely. It is highly desirable that the acti 2,924,620 vating group be easily and economically removed after it has served its purpose. Even with the activating PROCESS FOR THE PREPARATION OF N-acetyl group, the reaction is rather slow, about 20 to DIPHENYLAMINES 24 hours at elevated temperatures being required to Robert K. Miller, New Castle, Del., assignor to E. I. du achieve practical yields of the product of the condensation. Pont de Nemonrs and Company, Wilmington, Del., a It is an object of the present invention to utilize corporation of Delaware formanilides in the Ullmann condensation with aryl halides wherein the diarylamine is directly recoverable N0 Drawing. Application March 30, 1959 10 from the reaction mass. Serial No. 802,664 it is a further object of this invention to eliminate the 5 Claims. (Cl. 260-576) need for prolonged hydrolysis of the reaction product obtained from N-acetyl primary aromatic amine and aryl The present invention is directed to a novel method halide. ' for producing diphenylamines; this invention is particu 15 It is a further object of this invention to produce un larly useful in the preparation of unsymmetrical diphenyl expectedly high yields of diarylamine. amines. It is a speci?c object of the present invention to pro This application is a continuation-in-part of copending vide a simpli?ed and economically practical Ullmann applications Serial No. 592,732, ?led June 21, 1956 and condensation method for preparing 3-chlorodiphenyl application Serial No. -

Method for the Synthesis of Iodobenzene
Europaisches Patentamt European Patent Office (Tj) Publication number: 0 183 579 Office europeen des brevets A1 © EUROPEAN PATENT APPLICATION (£) Application number: 85401990.8 © Int. CI.4: C 07 C 25/02 C 07 C 17/156 © Date of filing: 14.10.85 © Priority: 16.10.84 IT 2316984 ©Applicant: MONTEDiPE S.p.A. 31, Foro Buonaparte MilanllT) © Date of publication of application: 04.06.86 Bulletin 86/23 © Inventor: Paparatto, Giuseppe 7 via Vasari © Designated Contracting States: Cinisello Balsamo (Milan)dT) BE DE FR GB IT NL Q2) Inventor: Saetti, Marco 73 via Pentapoli 1-96010 Prioio (SiracusaHIT) © Representative: Hirsch, Marc-Roger 34 rue de Bassano F-75008 Paris(FR) © Method for the synthesis of iodobenzene. A method for the synthesis of iodobenzene by iodina- tion, in the gaseous phase, of benzene with iodine, in the presence of a zeolitic catalyst, selected from the group comprising the zeolites of X type or of Y type, characterized in that said iodination is an oxidative one and that said zeolites are in a form different from the acid (H) form. The invention concerns a method for the synthesis of iodobenzene by iodination in the gaseous phase of benzene with iodine. The synthesis of iodobenzene starting from benzene and iodine is usually carried out in a liquid phase, in the presence of an oxidative agent; preferably the oxidative agent consists of nitric acid (see Japanese patent 58/77830, U.S.S.R. patent 453392 and an article of Datta R. L. and Chatterjee N. R. on the J. Am. Chem. Soc., 39, 437, 1917). -

Preparationbehaviorcertainhalo
:O: UBRAR.YOF THE ' ' 00: ' °UNIVERSIlY OF MISSOURI . '. ~ . ..... .. '\ THE PREPARATION AND BEHAVIOR OF CERTAIN HALOGEN AND NITRO DERIVATIVES OF BEnZENE by Harry Fagan Yancey, A. B. 'II • • • 10 . ~ , " . - " .. .. .. ... .. ... ___..... .:J. .. ..:...;..: .. .:. .. ) . "" . ... .": ... ~ ... 10 ', ..... -. SUBMITTED IN PARTIAL FULFILLMENT OF THE RE QUIREMENTS FOR THE DEGREE OF MASTER OF ARTS in the GRADUATE SCHOOL of the UNIVERSITY OF MISSOURI 1915 TABLE OF CONTENTS Page I Introduction 1- 4 II Experimental Part 5-18 1. Action of Sodium on Symmetrical Tribromo- trinitrobenzene. 5 2. Action of Sodium on l-Iodo-2.4,6-Tribromo- benBene. 5 3. Action of Sodium on Symmetrical Tribromo- iodobenzene and Ethyl Iodide. 8 4. Aotion of Sodium on Symmetrical Tribromo- benzene. 9 5. Prep'aration of l-Iodo-2,6-Dibromo-4-Ui- trobenzene. 10 6. Properties of 1-Iodo-2,6-Dibromo-4-Nitro- benzene. 11 7. Action of Sodium Ethylate on l-Iodo-2,6-Di- bromo-4-Nitrobenzene. 12 8. Preparation of l-Iodo-2,6-Dibromo-4,5-Dini- trobenzene. 13 9. Properties of l-Iodo-2,6-Dibromo-4,5-Dini- trobenzene. 13 10. Preparation of l-Chloro-2,6-Dibromo-4-Ni- trobenzene. 14 11. Action of Nitric Acid on 1-Iodo-2,4,6-Tri- bromobenzene. 17 12. Properties of l-Chloro-2,6-Dibromo-4-Nitro- benzene. 16 13. Preparation of l-Chloro-2,6-Dibromo-4,5-Di- nitrobenzene. 16 14. Properties of l-Ch1oro-2,6-Dibromo-4,5-Di- nitrobenzene. 16 III Discussion of Results 19-21 INTRODUCTION The common method for the preparation of diphenyl 1 and alkyl benzene derivatives is that of ~ittig. -

Efficient C-O and C-N Bond Forming Cross-Coupling Reactions Catalyzed by Core-Shell Structured
Efficient C-O and C-N bond forming cross-coupling reactions catalyzed by core-shell structured Cu/Cu2O nanowires Thesis by Ahmed Elshewy In Partial Fulfillment of the Requirements For the Degree of Master of Science King Abdullah University of Science and Technology, Thuwal, Kingdom of Saudi Arabia December 2013 2 The thesis of Ahmed Elshewy is approved by the examination committee. Committee Chairperson: Prof. Kuo-Wei Huang, Assistant Professor of Chemical Sciences, Division of Physical Sciences and Engineering Committee Member: Prof. Jorg Eppinger, Assistant Professor of Chemical Sciences, Division of Physical Sciences and Engineering Committee Member: Prof. Zhiping Lai, Associate Professor of Chemical and Biological Engineering, Division of Physical Sciences and Engineering 3 ABSTRACT Efficient C-O and C-N bond forming cross-coupling reactions catalyzed by core-shell structured Cu/Cu2O nanowires Ahmed Elshewy Oxygen and Nitrogen containing compounds are of utmost importance due to their interesting and diverse biological activities. The construction of the C-O and C–N bonds is of significance as it opens avenues for the introduction of ether and amine linkages in organic molecules. Despite significant advancements in this field, the construction of C-O and C–N bonds is still a major challenge for organic chemists, due to the involvement of harsh reaction conditions or the use of expensive catalysts or ligands in many cases. Thus, it is a challenge to develop alternative, milder, cheaper and more reproducible methodologies for the construction of these types of bonds. Herein, we introduce a new efficient ligand free catalytic system for C-O and C-N bond formation reactions. -
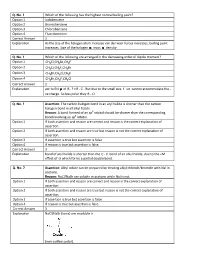
Q. No. 3 Which of the Following Has the Highest Normal Boiling Point
Q. No. 3 Which of the following has the highest normal boiling point? Option 1 Iodobenzene Option 2 Bromobenzene Option 3 Chlorobenzene Option 4 Fluorobenzene Correct Answer 1 Explanation As the size of the halogen atom increase van der waal forces increases, boiling point increases. Size of the halogen mass density. Q. No. 4 Which of the following are arranged in the decreasing order of dipole moment? Option 1 CH3 Cl,CH 3 Br,CH 3 F Option 2 CH3 Cl,CH 3 F,CH 3 Br Option 3 CH3 Br,CH 3 Cl,CH 3 F Option 4 CH3 Br,CH 3 F,CH 3 Cl Correct Answer 2 Explanation acc to EN of RF> RCl . But due to the small size. F ion cannot accommodate the ve charge. So less polar they R Cl . Q. No. 5 Assertion : The carbon halogen bond in an aryl halid e is shorter than the carbon halogen bond in all alkyl halide. Reason : A bond formed of an sp 3 orbital should be shorter than the corresponding bond involving an sp 2 orbital. Option 1 If both assertion and reason are correct and reason is the correct explanation of assertion. Option 2 If both assertion and reason are true but reason is not the correct explanation of assertion. Option 3 If assertion is true but assertion is false. Option 4 If reason is true but assertion is false. Correct Answer 3 Explanation bond of aryl halide is shorter than the C X bond of an alkyl halide, due to the +M effect of X which forms a partial double bond. -

Ultrafast REMPI in Benzene and the Monohalobenzenes Without the Focal Volume Effect Timothy D
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln C.J.G.J. Uiterwaal Publications Research Papers in Physics and Astronomy 2011 Ultrafast REMPI in benzene and the monohalobenzenes without the focal volume effect Timothy D. Scarborough University of Nebraska-Lincoln, [email protected] James Strohaber Texas A & M University - College Station, [email protected] David B. Foote University of Nebraska-Lincoln Collin J. McAcy University of Nebraska-Lincoln, [email protected] Cornelis J. Uiterwaal University of Nebraska-Lincoln, [email protected] Follow this and additional works at: http://digitalcommons.unl.edu/physicsuiterwaal Part of the Atomic, Molecular and Optical Physics Commons, Biological and Chemical Physics Commons, and the Physical Chemistry Commons Scarborough, Timothy D.; Strohaber, James; Foote, David B.; McAcy, Collin J.; and Uiterwaal, Cornelis J., "Ultrafast REMPI in benzene and the monohalobenzenes without the focal volume effect" (2011). C.J.G.J. Uiterwaal Publications. 20. http://digitalcommons.unl.edu/physicsuiterwaal/20 This Article is brought to you for free and open access by the Research Papers in Physics and Astronomy at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in C.J.G.J. Uiterwaal Publications by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. View Article Online / Journal Homepage / Table of Contents for this issue PCCP Dynamic Article Links Cite this: Phys. Chem. Chem. Phys., 2011, 13, 13783–13790 www.rsc.org/pccp PAPER Ultrafast REMPI in benzene and the monohalobenzenes without the focal volume effect Timothy D. Scarborough,*a James Strohaber,b David B. Foote,a Collin J. McAcya and Cornelis J.