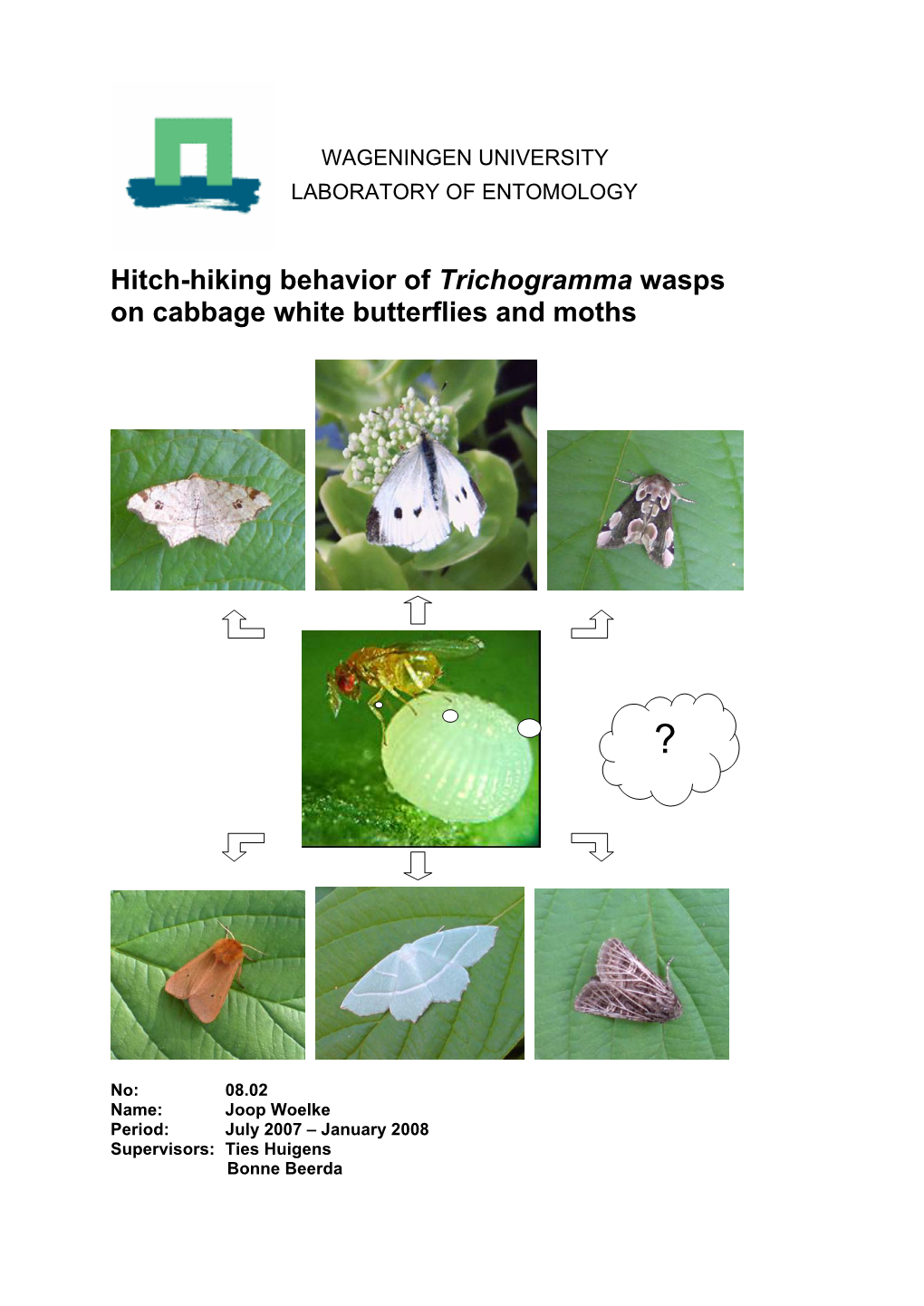
Hitch-Hiking Behavior of Trichogramma Wasps on Cabbage White Butterflies and Moths
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Entomofauna Ansfelden/Austria; Download Unter
©Entomofauna Ansfelden/Austria; download unter www.biologiezentrum.at Entomofauna ZEITSCHRIFT FÜR ENTOMOLOGIE Band 28, Heft 28: 377-388 ISSN 0250-4413 Ansfelden, 30. November 2007 Phytophagous Noctuidae (Lepidoptera) of the Western Black Sea Region and their ichneumonid parasitoids Z. OKYAR & M. YURTCAN Abstract Eleven agricultural and silviculturally important species of Noctuidae and their parasitoids were determined in 33 localities from the Western Black Sea region between 2001 and 2004. The ichneumonid biological control agents Enicospilus ramidulus, Barylypa amabilis and Itoplectis alternans were obtained by rearing the host larvae. K e y w o r d s : Lepidoptera, Noctuidae, Hymenoptera, Ichneumonidae, parasitoidism, Western Black Sea Region, Turkey Zusammenfassung 11 land- und forstwirtschaftlich bedeutende Noctuidae-Arten einschließlich ihrer Parasitoide aus 33 Standorten des Gebietes des westlichen Schwarzen Meeres wurden im Zeitraum 2001 bis 2004 studiert. Ichneumonidae der Arten Enicospilus ramidulus, Barylypa amabilis and Itoplectis alternans konnten durch Aufzucht der Wirtslarven festgestellt werden. 377 ©Entomofauna Ansfelden/Austria; download unter www.biologiezentrum.at Introduction The Noctuidae is the largest family of the Lepidoptera. Larvae of some species are par- ticularly harmful to agricultural and silvicultural regions worldwide. Consequently, for years intense efforts have been carried out to control them through chemical, biological, and cultural methods (LIBURD et al. 2000; HOBALLAH et al. 2004; TOPRAK & GÜRKAN 2005). In the field, noctuid control is often carried out by parasitoid wasps (CHO et al. 2006). Ichneumonids are one of the most prevalent parasitoid groups of noctuids but they also parasitize on other many Lepidoptera, Coleoptera, Hymenoptera, Diptera and Araneae (KASPARYAN 1981; FITTON et al. 1987, 1988; GAULD & BOLTON 1988; WAHL 1993; GEORGIEV & KOLAROV 1999). -

Heathland 700 the Park & Poor's Allotment Species List
The Park & Poor's Allotment Bioblitz 25th - 26th July 2015 Common Name Scientific Name [if known] Site recorded Fungus Xylaria polymorpha Dead Man's Fingers Both Amanita excelsa var. excelsa Grey Spotted Amanita Poor's Allotment Panaeolus sp. Poor's Allotment Phallus impudicus var. impudicus Stinkhorn The Park Mosses Sphagnum denticulatum Cow-horn Bog-moss Both Sphagnum fimbriatum Fringed Bog-moss The Park Sphagnum papillosum Papillose Bog-moss The Park Sphagnum squarrosum Spiky Bog-moss The Park Sphagnum palustre Blunt-leaved Bog-moss Poor's Allotment Atrichum undulatum Common Smoothcap Both Polytrichum commune Common Haircap The Park Polytrichum formosum Bank Haircap Both Polytrichum juniperinum Juniper Haircap The Park Tetraphis pellucida Pellucid Four-tooth Moss The Park Schistidium crassipilum Thickpoint Grimmia Poor's Allotment Fissidens taxifolius Common Pocket-moss The Park Ceratodon purpureus Redshank The Park Dicranoweisia cirrata Common Pincushion Both Dicranella heteromalla Silky Forklet-moss Both Dicranella varia Variable Forklet-moss The Park Dicranum scoparium Broom Fork-moss Both Campylopus flexuosus Rusty Swan-neck Moss Poor's Allotment Campylopus introflexus Heath Star Moss Both Campylopus pyriformis Dwarf Swan-neck Moss The Park Bryoerythrophyllum Red Beard-moss Poor's Allotment Barbula convoluta Lesser Bird's-claw Beard-moss The Park Didymodon fallax Fallacious Beard-moss The Park Didymodon insulanus Cylindric Beard-moss Poor's Allotment Zygodon conoideus Lesser Yoke-moss The Park Zygodon viridissimus Green Yoke-moss -

St Julians Park Species List, 1984 – 2003
St Julians Park Species List, 1984 – 2003 Fungi Species Common Name Date recorded Scleroderma citrinum Common Earth Ball 19/09/99 Amillaria mellea Honey Fungus 19/09/99 Hypholoma sublateridium Brick Caps 19/09/99 Piptoporus belulinus Birch Polypore 19/09/99 Lycoperdon perlatum Common Puffball 19/09/99 Coriolus versicolor Many-Zoned Polypore 19/09/99 Boletus erythropus - 19/09/99 Lactarius quietus Oak/Oily Milk Cap 19/09/99 Russula cyanoxantha The Charcoal Burner 19/09/99 Amanita muscaria Fly Agaric 19/09/99 Laccaria laccata Deceiver 19/09/99 Lepidoptera Species Common Name Date recorded Melanargia galathea Marbled White 1992/3 Venessa cardui Painted Lady 1992/3 Thymelicus sylvestris Small Skipper 1992/3, 06/06/98 Ochlodes venata Large Skipper 1992/3, 06/06/98 Pararge aegeria Speckled Wood 1992/3, 06/06/98 Venessa atalanta Red Admiral 1992/3 Aglais urticae Small Tortoiseshell 1992/3 Polyommatus icarus Common Blue 1992/3, 06/06/98 Pyronia tithonus Gamekeeper 1992/3 Maniola jurtina Meadow Brown 1992/3, 06/06/98 Aphantopus hyperantus Ringlet 1992/3, 06/06/98 Inachis 10 Peacock 1992/3, 23/03/00 Polygonia C-album Comma 1992/3, 23/03/00 Anthocaris cardamines Orange Tip 1992/3 Noctua pronuba Large Yellow Underwing 06/06/98 Pieris brassicae Large White 06/06/98 Zygaena trifolii 5 Spot Burnet 06/06/98 Diboba caeruleocephala Figure of Eight 22/10/99 Xanthia aurago Barred Sallow 22/10/99 Chloroclysta truncate Common Marbled Carpet 22/10/99 Epirrata dilutata November Moth 22/10/99 Epirrata chrysti Pale November Moth 22/10/99, 07/11/99 Chloroclysta -

Lockerbie Wildlife Trust Eskrigg Reserve June 2017 News Bulletin
Lockerbie Wildlife Trust (www.lockerbie-wildlife-trust.co.uk) Eskrigg Reserve Scottish Charity No: SC 005538 June 2017 News Bulletin 1. Eskrigg Pond on the 27th June, during a heavy rain shower (JR). JR 2. Confirmed wildlife sightings at the Reserve in June. a. Birds Blackbird, Blackcap, Blue Tit, Bullfinch, Buzzard, Carrion Crow, Chaffinch, Chiffchaff, Coal Tit, Collared Dove, Crossbill, Dunnock, Goldfinch, Great Spotted Woodpecker, Great Tit, Grey Heron, Grey Wagtail, House Martin, House Sparrow, Jackdaw, Jay, Little Grebe, Long-tailed Tit, Mallard, Mandarin, Mistle Thrush, Moorhen, Mute Swan, Nuthatch, Oystercatcher, Pheasant, Pied Wagtail, Raven, Robin, Siskin, Song Thrush, Sparrowhawk, Starling, Swallow, Swift, Tawny Owl, Treecreeper, Tree Pipit, Tree Sparrow, Willow Warbler, Wood Tawny Owlets (JH) Pigeon, Wood Warbler, Wren, Yellowhammer. b. Mammals Bank Vole, Mole, Rabbit, Red Squirrel, Roe Deer, Wood Mouse. c. Fish, Amphibians and Reptiles Minnow, Stickleback, Common Frog, Common Toad, Common Lizard. Red Squirrel (PW) Photographs by Jim Halliday (JH), Jim Rae (JR), Paul Wharton (PW) 1!! Jim Rae (Eskrigg Reserve Manager) Address: Carradale, 12 Douglas Terrace, Lockerbie, Dumfries and Galloway, DG11 2DZ. Home Tel.: 01576 203 314 / Mobile No.: 07739 987 009 Email: [email protected] 3. June Photo Gallery Row 1: Pheasant poult (PW), Mute Swan cygnet (PW), Grey Heron (PW). Row 2: Female Azure Damselfy (JR), Nettle-tap (JR), Green-legged Sawfly (JR). Row 3: Russian Comfrey (JR), Marsh Cinquefoil (JR), Pyrenean Lilly (JR). Row 4: Tawny Owl - Mother, (JR), Tawny Owlet - elder offspring (JR), Tawny Owlet - younger offspring (JR) Row 5: Grey Dagger (JR), Beautiful Snout (JR), A Yellow-barred Longhorn (JR). -

Attraction of Trichogramma Wasps to Brassica Nigra Plants Induced by Lepidopteran Eggs
Attraction of Trichogramma wasps to Brassica nigra plants induced by lepidopteran eggs Ilich A. Figueroa Supervisors: Nina Fatouros, Ties Huigens Examiner: Marcel Dicke MSc. Minor Thesis– ENT-80424 Report no. 010.27 MSc Plant Science Program Laboratory of Entomology Wageningen University December, 2010 Abstract Plants possess a broad spectrum of defense mechanisms against herbivore attack. The black mustard Brassica nigra, is able to display early defense mechanism against egg infestation by pierid butterflies. This plant shows induced direct defense through hypersensitive response (HR), which kills the eggs as well as indirect defense by the emission of egg-induced plant volatiles that attract egg parasitoids such as Trichogramma wasp. In this study, I investigate whether B. nigra plants infested by the small cabbage white butterfly (Pieris rapae) or the cabbage moth (Mamestra brassicae) express both kind of defense strategies, and whether plants expressing HR still attract Trichgramma wasps in the laboratory and in nature. Tests in the y-tube olfactometer showed that volatiles of plants infested with P. rapae eggs 24h after egg deposition were attractive to the egg parasitoid Trichogramma brassicae when tested against volatiles of uninfested plants. All tested P. rapae-infested plants expressed HR 24h after oviposition. In contrast, plants infested with M. brassicae eggs did not express HR. Volatiles of M. brassicae egg-infested plants were attractive to T. brassicae only when tested against clean air but not when tested against volatiles of uninfested plants. In nature, 77% of the P. rapae eggs collected from HR+ B. nigra plants died, whereby 37% because of Trichogramma parasitism. Eggs collected on HR- B. -

Invasive Plants Established in the United States That Are Found In
Stellaria media Common chickweed Introduction The genus Stellaria contains approximately 190 species, occurring primarily in the temperate regions. Sixty-four species have been reported in China[12]. Taxonomy Order: Centrospermae Suborder: Caryophyllineae Family: Caryophyllaceae Subfamily: Alsinoideae Vierh. Tribe: Alsineae Pax Subtribe: Stellarinae Aschers. et apex and attenuate or subcordate base, stage of crop growth of wheat, rape and Graebn. lower leaf is petioled. The inflorescence some vegetables. It is also poisonous [39, 108, 187] Genus: Stellaria L. is a terminal cyme. The sepals are, to poultry . Section: Stellaria ovate lanceolate, about 4 mm in length, Subsection: Stellaria slightly obtuse or suborbicular apically Related Species Series: Petiolares Fenal and covered with short glandular hairs S. media var. micrantha (Hayata) T. S. Species: Stellaria media (L.) outside. Shorter than sepals, each Liu & S. S. Ying, native to Taiwan, is Cyr. petal is white, oblong, nearly bisected. a perennial herb. Its petals are nearly Shorter than petals, stamens are 3-5 equal to sepals, which are 2-2.5 mm Description with 3 linear styles. Slightly longer long, whereas S. media var. media has [12] Stellaria media is an annual or biennual than the persistent calyx, capsules are longer sepals and shorter petals . S. herb that can reach 10-30 cm in height. ovate, 6-lobed apically. The flowers media and five additional members The stem is light purplish red with one bloom from June to July and followed of the genus Stellaria are regarded as or two rows of hairs on the surface, and by fruits in July through August. The unwelcome plants in China. -

Effects of Artificial Diets and Floral Nectar on Parasitization
Türk. entomol. derg., 2017, 41 (1): 53-60 ISSN 1010-6960 DOI: http://dx.doi.org/10.16970/ted.68668 E-ISSN 2536-491X Original article (Orijinal araştırma) Effects of artificial diets and floral nectar on parasitization performance of Trichogramma brassicae Bezdenko, 1968 (Hymenoptera: Trichogrammatidae)1 Yapay besin ve bitki nektarının Trichogramma brassicae Bezdenko, 1968 (Hymenoptera: Trichogrammatidae)’nin parazitleme performansına etkileri Nihal ÖZDER2* Şeyda DEMİRTAŞ2 Summary This study was conducted to determine whether various food resources enhanced the longevity and fecundity of the egg parasitoid Trichogramma brassicae Bezdenko, 1968 (Hymenoptera: Trichogrammatidae) under laboratory conditions (25°C, 65% RH, 16L:8D h photoperiod) at Laboratory of Biological Control, Department of Plant Protection, Agriculture Faculty, Namık Kemal University in 2014. Newly hatched female wasps were fed on Ephestia kuehniella Zeller, 1879 (Lepidoptera: Pyralidae) eggs with either honey, grape molasses and royal jelly as a main food, alone or double combination of this main foods or supplemented with resin (derived from plants), acacia nectar, Paulownia nectar, red tulip nectar, yellow asphodel nectar, apple syrup, liquid of E. kuehniella eggs or mashed E. kuehniella larvae. Trichogramma brassicae, females that were fed on honey and acacia nectar (17.47 d), honey + apple syrup (17.20 d), honey (16.93 d) and honey + Paulownia nectar (16.60 d) lived significantly longer than females that fed on other floral nectars and artificial diets. Females were fed on royal jelly + mashed E. kuehniella larvae (1.40 d) had the shortest longevity. Trichogramma brassicae females that were fed on honey (106.8 eggs), honey + acacia nectar (105.4 eggs), Paulownia nectar (103.13 eggs) parasitized significantly more hosts than females that fed on other floral nectars and artificial diets. -

Research Techniques in Animal Ecology
Research Techniques in Animal Ecology Methods and Cases in Conservation Science Mary C. Pearl, Editor Methods and Cases in Conservation Science Tropical Deforestation: Small Farmers and Land Clearing in the Ecuadorian Amazon Thomas K. Rudel and Bruce Horowitz Bison: Mating and Conservation in Small Populations Joel Berger and Carol Cunningham, Population Management for Survival and Recovery: Analytical Methods and Strategies in Small Population Conservation Jonathan D. Ballou, Michael Gilpin, and Thomas J. Foose, Conserving Wildlife: International Education and Communication Approaches Susan K. Jacobson Remote Sensing Imagery for Natural Resources Management: A First Time User’s Guide David S. Wilkie and John T. Finn At the End of the Rainbow? Gold, Land, and People in the Brazilian Amazon Gordon MacMillan Perspectives in Biological Diversity Series Conserving Natural Value Holmes Rolston III Series Editor, Mary C. Pearl Series Advisers, Christine Padoch and Douglas Daly Research Techniques in Animal Ecology Controversies and Consequences Luigi Boitani and Todd K. Fuller Editors C COLUMBIA UNIVERSITY PRESS NEW YORK Columbia University Press Publishers Since 1893 New York Chichester, West Sussex Copyright © 2000 by Columbia University Press All rights reserved Library of Congress Cataloging-in-Publication Data Research techniques in animal ecology : controversies and consequences / Luigi Boitani and Todd K. Fuller, editors. p. cm. — (Methods and cases in conservation science) Includes bibliographical references (p. ). ISBN 0–231–11340–4 (cloth : alk. paper)—ISBN 0–231–11341–2 (paper : alk. paper) 1. Animal ecology—Research—Methodology. I. Boitani, Luigi. II. Fuller, T. K. III. Series. QH541.2.R47 2000 591.7′07′2—dc21 99–052230 ϱ Casebound editions of Columbia University Press books are printed on permanent and durable acid-free paper. -

Harmonization of Regulations for Invertebrate Biocontrol Agents in Europe: Progress, Problems and Solutions J
J. Appl. Entomol. MINI REVIEW Harmonization of regulations for invertebrate biocontrol agents in Europe: progress, problems and solutions J. Bale School of Biosciences, University of Birmingham, Edgbaston, Birmingham, UK Keywords Abstract biological control, environmental risk assessment, Europe, regulation The use of non-native invertebrate biological control agents (IBCAs) in Europe is not covered by a Directive equivalent to that which regulates Correspondence biocontrol with microorganisms or the genetic modification of crop Jeffrey Bale (corresponding author), School of plants. Regulation is at the discretion of individual member states and Biosciences, University of Birmingham, largely derived from national legislation on pesticides, plant health or Edgbaston, Birmingham B15 2TT, UK. environmental protection. There is no EU country with regulation of E-mail: [email protected] IBCAs that requires information on the microbial symbiont content of Received: September 29, 2010; accepted: candidate species, and in the absence of horizontal transfer under natu- December 23, 2010. ral conditions, this policy is unlikely to change. Although there have been few reported negative effects linked to the import and release of doi: 10.1111/j.1439-0418.2011.01611.x IBCAs, a number of countries have introduced or revised their regula- tory frameworks in recent years. This article reviews major develop- ments in the regulation and environmental risk assessment (ERA) of IBCAs in Europe over the last 10 years including: the fragmented pat- tern of regulation between countries, variation in information require- ments for release licences, format and methods of ERA for different taxonomic groups of IBCAs, use and updating of the European Plant Protection Organisation Positive List, sources of expert advice on ERA data, communication between IBCA regulators, and options for the provision of international leadership to coordinate regulatory and ERA-related issues with IBCA-based biocontrol in Europe. -

Nachtvlinderonderzoek in De Wassenaarse Duinen
Nachtvlinderonderzoek in de Wassenaarse duinen Wouter Moerland Neeltje van Zuytbrouckhof 50 2311 WD Leiden [email protected] In de duinen bij Wassenaar worden sinds 2004 nachtvlinders gevangen. De eerste vlinderinventarisaties vonden plaats als onderdeel van de veldcursus ‘dierecologie’ aan de Leidse universiteit. Tegenwoordig zijn er particuliere initiatieven, zoals excursies van de NJN of de Leidse biologenclub. Kleinschalig vlinder- onderzoek toonde al de aanzienlijke vlinderdiversiteit in de Hollandse duinen: resultaten tot 2006 wor- den gepresenteerd door Van Alphen (2006). Sindsdien is het onderzoek voortgezet, met enkele grote inventarisaties. In juli 2007 en 2008 is in verband met de toen nieuwe website www.microlepidoptera.nl de omgeving van veldstation De Klip grondig bemonsterd. Ook buiten dit ‘vlinderhoogseizoen’ is volop gevlinderd, van maart tot in de december. Op basis van deze aanvullende vangsten presenteert dit artikel een herziene en geactualiseerde soortenlijst van nachtvlinders. Het geeft een overzicht van de resultaten van zeven jaar nachtvlinderen in de Wassenaarse duinen. Onderzoek naar nachtvlinders Vlinders (Lepidoptera) vormen in Nederland een diverse groep insecten: ongeveer 2200 soorten zijn er gevestigd (Noordijk et al. 2010). Een niet-wetenschappelijk maar pragmatisch onderscheid wordt gemaakt tussen de ‘grote’ en ‘kleine’ vlinders. Een derde van die 2200 soorten wordt gerekend tot die grote vlinders, de macrolepidoptera (macro’s), inclusief dagvlinders. Het overige deel, de kleine vlinders, wordt microlepidoptera (micro’s) genoemd. Alle vlinders die niet tot de dagvlinders worden gerekend, bestempelt men als nachtvlinders. Figuur 1. Directe omgeving De Klip in de schemering – op de bunker een nachtvlinderlaken. Foto WM 16 Holland’s Duinen nr 57, april 2011 Nachtvlinderonderzoek in de Wassenaarse duinen Veel van de nachtvlinders vliegen ’s nachts of in de schemering. -

Additions, Deletions and Corrections to An
Bulletin of the Irish Biogeographical Society No. 36 (2012) ADDITIONS, DELETIONS AND CORRECTIONS TO AN ANNOTATED CHECKLIST OF THE IRISH BUTTERFLIES AND MOTHS (LEPIDOPTERA) WITH A CONCISE CHECKLIST OF IRISH SPECIES AND ELACHISTA BIATOMELLA (STAINTON, 1848) NEW TO IRELAND K. G. M. Bond1 and J. P. O’Connor2 1Department of Zoology and Animal Ecology, School of BEES, University College Cork, Distillery Fields, North Mall, Cork, Ireland. e-mail: <[email protected]> 2Emeritus Entomologist, National Museum of Ireland, Kildare Street, Dublin 2, Ireland. Abstract Additions, deletions and corrections are made to the Irish checklist of butterflies and moths (Lepidoptera). Elachista biatomella (Stainton, 1848) is added to the Irish list. The total number of confirmed Irish species of Lepidoptera now stands at 1480. Key words: Lepidoptera, additions, deletions, corrections, Irish list, Elachista biatomella Introduction Bond, Nash and O’Connor (2006) provided a checklist of the Irish Lepidoptera. Since its publication, many new discoveries have been made and are reported here. In addition, several deletions have been made. A concise and updated checklist is provided. The following abbreviations are used in the text: BM(NH) – The Natural History Museum, London; NMINH – National Museum of Ireland, Natural History, Dublin. The total number of confirmed Irish species now stands at 1480, an addition of 68 since Bond et al. (2006). Taxonomic arrangement As a result of recent systematic research, it has been necessary to replace the arrangement familiar to British and Irish Lepidopterists by the Fauna Europaea [FE] system used by Karsholt 60 Bulletin of the Irish Biogeographical Society No. 36 (2012) and Razowski, which is widely used in continental Europe. -

Barrowhill, Otterpool and East Stour River)
Folkestone and Hythe Birds Tetrad Guide: TR13 D (Barrowhill, Otterpool and East Stour River) The tetrad TR13 D is an area of mostly farmland with several small waterways, of which the East Stour River is the most significant, and there are four small lakes (though none are publically-accessible), the most northerly of which is mostly covered with Phragmites. Other features of interest include a belt of trees running across the northern limit of Lympne Old Airfield (in the extreme south edge of the tetrad), part of Harringe Brooks Wood (which has no public access), the disused (Otterpool) quarry workings and the westernmost extent of Folkestone Racecourse and. The northern half of the tetrad is crossed by the major transport links of the M20 and the railway, whilst the old Ashford Road (A20), runs more or less diagonally across. Looking south-west towards Burnbrae from the railway Whilst there are no sites of particular ornithological significance within the area it is not without interest. A variety of farmland birds breed, including Kestrel, Stock Dove, Sky Lark, Chiffchaff, Blackcap, Lesser Whitethroat, Yellowhammer, and possibly Buzzard, Yellow Wagtail and Meadow Pipit. Two rapidly declining species, Turtle Dove and Spotted Flycatcher, also probably bred during the 2007-11 Bird Atlas. The Phragmites at the most northerly lake support breeding Reed Warbler and Reed Bunting. In winter Fieldfare and Redwing may be found in the fields, whilst the streams have attracted Little Egret, Snipe and, Grey Wagtail, with Siskin and occasionally Lesser Redpoll in the alders along the East Stour River. Corn Bunting may be present if winter stubble is left and Red Kite, Peregrine, Merlin and Waxwing have also occurred.