The Format of the IJOPCM, First Submission
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

AGS Seed List No 69 2020
Seed list No 69 2020-21 Garden Collected Seed 1001 Abelia floribunda 1057 Agrostemma githago 1002 Abies koreana 1058 Albuca canadensis (L. -

Karyological Studies on Iris Japonica Thunb. and Its Allies1) 2) by K
180 Cytologia 10 Karyological Studies on Iris japonica Thunb. and Its Allies1) 2) By K. Yasui Tokyo Imperial University (With 8 text figures) Receic ed MTV 28, 1939 Introduction There are several karyological investigations on Iris japonica. KAZAO (1928) considered the sporophyte of this species as an auto triploid having 54 chromosomes. But SIMONET (1932, 1934) re ported I. japonica as a diploid plant with 34 chromosomes in its root-tip cells, and considered I. japonica var aphrodite as a hyper triploid and interpreted the chromosome constitution as 2n=51+3, the latter 3 chromosomes being considered as derived from 3 of 51 chromosomes in I. japonica by fragmentation which occurred att their median constrictions. The karyological investigation in I. japonica and the other 2 allied species, which were kindly put at my deposal by Dr. S. IMA MURA of Kyoto Imp. University, gave me some results different from those of the previous authors as is described below. Material and Method In the fixing of the root-tip cells both NAVASHIN's and LEWITSKY's solutions were used. When fixed in the latter, the chromosomes were found longer and slender than when fixed in the former solution, but there was no essential difference in the distribu tion of chromosomes in the equatorial plate. NEWTON'S gentian violet staining was generally adopted. Materials for the study of meiosis were fixed mostly with NAVASHIN'S fluid and stained with HEIDENHAIN'S iron-alum haematoxylin. Acetocarmine smears were also tried in the study of the pollen mother cells. Drawings were made with a camera lucida. -
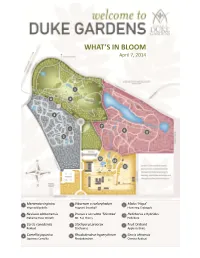
What's in Bloom
WHAT’S IN BLOOM April 7, 2014 5 4 6 2 7 1 9 8 3 12 10 11 1 Mertensia virginica 5 Viburnum x carlcephalum 9 Malus ‘Hopa’ Virginia Bluebells Fragrant Snowball Flowering Crabapple 2 Neviusia alabamensis 6 Prunus x serrulata ‘Shirotae’ 10 Helleborus x hybridus Alabama Snow Wreath Mt. Fuji Cherry Hellebore 3 Cercis canadensis 7 Stachyurus praecox 11 Fruit Orchard Redbud Stachyurus Apple cultivars 4 Camellia japonica 8 Rhododendron hyperythrum 12 Cercis chinensis Japanese Camellia Rhododendron Chinese Redbud WHAT’S IN BLOOM April 7, 2014 BLOMQUIST GARDEN OF NATIVE PLANTS Amelanchier arborea Common Serviceberry Sanguinaria canadensis Bloodroot Cornus florida Flowering Dogwood Stylophorum diphyllum Celandine Poppy Thalictrum thalictroides Rue Anemone Fothergilla major Fothergilla Trillium decipiens Chattahoochee River Trillium Hepatica nobilis Hepatica Trillium grandiflorum White Trillium Hexastylis virginica Wild Ginger Hexastylis minor Wild Ginger Trillium pusillum Dwarf Wakerobin Illicium floridanum Florida Anise Tree Trillium stamineum Blue Ridge Wakerobin Malus coronaria Sweet Crabapple Uvularia sessilifolia Sessileleaf Bellwort Mertensia virginica Virginia Bluebells Pachysandra procumbens Allegheny spurge Prunus americana American Plum DORIS DUKE CENTER GARDENS Camellia japonica Japanese Camellia Pulmonaria ‘Diana Clare’ Lungwort Cercis canadensis Redbud Prunus persica Flowering Peach Puschkinia scilloides Striped Squill Cercis chinensis Redbud Sanguinaria canadensis Bloodroot Clematis armandii Evergreen Clematis Spiraea prunifolia Bridalwreath -

Four New Early Spring-Flowering Evergreen Iris Cultivars
CULTIVAR AND GERMPLASM RELEASES HORTSCIENCE 55(1):103–105. 2020. https://doi.org/10.21273/HORTSCI14433-19 Dawn’ (‘Xiaodie’), ‘Butterflies in Bloom’ (‘Huadie’), and ‘Butterfly Veil’ (‘Dieyi’) by Four New Early Spring-flowering the American Iris Society in 2019. Evergreen Iris Cultivars Description Feng-yang Yu and Yue-e Xiao The selection process was conducted at the Conservation Nursery of the Shanghai Research Center, Shanghai Botanical Garden, Shanghai, 200231, China; Botanical Garden in Shanghai, China. For and Shanghai Engineering Research Center of Sustainable Plant Innovation, each of the four new cultivars, 30 clones were Shanghai 200231, China planted (4 cultivars · 30 clones) in Sept. 2016. The plants were grown in arrays with Lin Cheng 30 cm between plants in the experimental College of Biology and Environment, Nanjing Forest University, Nanjing field; they were irrigated and fertilized simi- 210037, China larly to other perennial herbs. All plants were grown at a forest edge where they received half Shu-cheng Feng and Lei-lei Zhang full-sunlight. Morphological characteristics in- Research Center, Shanghai Botanical Garden, Shanghai, 200231, China; cluding plant height, leaf length and width, and Shanghai Engineering Research Center of Sustainable Plant Innovation, flower color, flower diameter, inner perianth length and width, outer perianth length and Shanghai 200231, China width, and the flowering period of the total Additional index words. cultivar, flower period, Iris japonica, ornamental, selection population. Single flowers were evaluated for a randomized sample of 15 plants per cultivar. Leaf length and width were measured on the Iris, with its showy and colorful flowers, is from late March to mid-April in Shanghai third leaf from the top of each plant. -

Araliaceae.Pdf
ARALIACEAE 五加科 wu jia ke Xiang Qibai (向其柏 Shang Chih-bei)1; Porter P. Lowry II2 Trees or shrubs, sometimes woody vines with aerial roots, rarely perennial herbs, hermaphroditic, andromonoecious or dioecious, often with stellate indumentum or more rarely simple trichomes or bristles, with or without prickles, secretory canals pres- ent in most parts. Leaves alternate, rarely opposite (never in Chinese taxa), simple and often palmately lobed, palmately compound, or 1–3-pinnately compound, usually crowded toward apices of branches, base of petiole often broad and sheathing stem, stipules absent or forming a ligule or membranous border of petiole. Inflorescence terminal or pseudo-lateral (by delayed development), um- bellate, compound-umbellate, racemose, racemose-umbellate, or racemose-paniculate, ultimate units usually umbels or heads, occa- sionally racemes or spikes, flowers rarely solitary; bracts usually present, often caducous, rarely foliaceous. Flowers bisexual or unisexual, actinomorphic. Pedicels often jointed below ovary and forming an articulation. Calyx absent or forming a low rim, some- times undulate or with short teeth. Corolla of (3–)5(–20) petals, free or rarely united, mostly valvate, sometimes imbricate. Stamens usually as many as and alternate with petals, sometimes numerous, distinct, inserted at edge of disk; anthers versatile, introrse, 2- celled (or 4-celled in some non-Chinese taxa), longitudinally dehiscent. Disk epigynous, often fleshy, slightly depressed to rounded or conic, sometimes confluent with styles. Ovary inferior (rarely secondarily superior in some non-Chinese taxa), (1 or)2–10(to many)-carpellate; carpels united, with as many locules; ovules pendulous, 2 per locule, 1 abortive; styles as many as carpels, free or partially united, erect or recurved, or fully united to form a column; stigmas terminal or decurrent on inner face of styles, or sessile on disk, circular to elliptic and radiating. -

Pollen and Stamen Mimicry: the Alpine Flora As a Case Study
Arthropod-Plant Interactions DOI 10.1007/s11829-017-9525-5 ORIGINAL PAPER Pollen and stamen mimicry: the alpine flora as a case study 1 1 1 1 Klaus Lunau • Sabine Konzmann • Lena Winter • Vanessa Kamphausen • Zong-Xin Ren2 Received: 1 June 2016 / Accepted: 6 April 2017 Ó The Author(s) 2017. This article is an open access publication Abstract Many melittophilous flowers display yellow and Dichogamous and diclinous species display pollen- and UV-absorbing floral guides that resemble the most com- stamen-imitating structures more often than non-dichoga- mon colour of pollen and anthers. The yellow coloured mous and non-diclinous species, respectively. The visual anthers and pollen and the similarly coloured flower guides similarity between the androecium and other floral organs are described as key features of a pollen and stamen is attributed to mimicry, i.e. deception caused by the flower mimicry system. In this study, we investigated the entire visitor’s inability to discriminate between model and angiosperm flora of the Alps with regard to visually dis- mimic, sensory exploitation, and signal standardisation played pollen and floral guides. All species were checked among floral morphs, flowering phases, and co-flowering for the presence of pollen- and stamen-imitating structures species. We critically discuss deviant pollen and stamen using colour photographs. Most flowering plants of the mimicry concepts and evaluate the frequent evolution of Alps display yellow pollen and at least 28% of the species pollen-imitating structures in view of the conflicting use of display pollen- or stamen-imitating structures. The most pollen for pollination in flowering plants and provision of frequent types of pollen and stamen imitations were pollen for offspring in bees. -

Flower-Visiting by the Invasive Hornet Vespa Velutina Nigrithorax (Hymenoptera: Vespidae)
International Journal of Chemical, Environmental & Biological Sciences (IJCEBS) Volume 3, Issue 6 (2015) ISSN 2320–4087 (Online) Flower-Visiting by the Invasive Hornet Vespa Velutina Nigrithorax (Hymenoptera: Vespidae) Takatoshi Ueno1 subspecies on the basis of the external morphology, i.e., color Abstract—The Asian hornet or yellow-legged hornet Vespa and marking patterns [8]; the subspecies nigrithorax is natively velutina nigrithorax has recently been recorded as an invasive insect distributed in southern China and northern India. However, in evoking environmental, apicultural and medical problems. Many 2000’s, this subspecies was unintentionally introduced into aspects of the ecology, behavior and life history remain unknown, Europe and Korea, where it has been increasing the populations however. The present study focuses on flower-visiting by the hornet in and expanding the range [9—11]. More recently, V. velutina the field. The foraging behavior of V. velutina nigrithorax on and nigrithorax has been found established in Tsushima Island of around blooming plants was observed in Tsushima Island, Japan and Busan City, South Korea. The field observations confirmed that the Japan in 2013 [12, 13]. Further, a nest of this hornet was hornet fed on floral nectar of 27 plant species scattering among 15 discovered in Kitakyushu City, mainland Japan in 2015 [14]. families. Pollen feeding was not observed. In addition, it was Thus, the hornet has been extending its range in East Asia. frequently found that V. velutina nigrithorax flew and hovered around Vespa velutina nigrithorax is recognized as an invasive a patch of flowers to predate hymenopteran bees and dipteran flies. species [10, 11]. -

Overcoming the Barriers to Green Walls in Urban Areas of the UK
Overcoming the barriers to green walls in urban areas of the UK Thesis submitted in partial fulfilment for the degree of Doctor of Engineering Technologies for Sustainable Built Environments Centre School of the Built Environment Faye Thomsit-Ireland September 2018 Declaration: I confirm that this is my own work and the use of all material from other sources has been properly and fully acknowledged. Faye Thomsit-Ireland September 2018 i Abstract Green infrastructure is seen as a tool to mitigate a host of environmental challenges in urban areas. Vertical greening solutions such as direct greening are gaining popularity due to relatively low cost and the fact that they have a minimal ground footprint. There are still, however, a range of barriers to their uptake, including worries about potential wall damage (physically and via RH increase). This research had sponsors from multiple disciplines and as such covers a wide range of topics aimed at reducing barriers to installations of direct greening. The impact of several popular and widely-used plant species (Hedera helix (English ivy), Parthenocissus tricuspidata (Boston creeper), and Pileostegia viburnoides (climbing hydrangea)), on the internal/external temperature and relative humidity (RH) of replicated experimental model ‘buildings’ (three per plant species, plus bare buildings) was studied over two summers and winters. All the plant species reduced both the air temperature internally/externally during the summer daytimes by at least 1 oC (Hedera produced the greatest cooling effect internally and externally, 7.2 oC and 8.3 oC reduction, respectively). All plant species reduced the daily ‘variation’ (morning to afternoon) in external RH, and external and internal temperature during summer (Hedera reduced variation most and Pileostegia least). -

Iris Sibirica and Others Iris Albicans Known As Cemetery
Iris Sibirica and others Iris Albicans Known as Cemetery Iris as is planted on Muslim cemeteries. Two different species use this name; the commoner is just a white form of Iris germanica, widespread in the Mediterranean. This is widely available in the horticultural trade under the name of albicans, but it is not true to name. True Iris albicans which we are offering here occurs only in Arabia and Yemen. It is some 60cm tall, with greyish leaves and one to three, strongly and sweetly scented, 9cm flowers. The petals are pure, bone- white. The bracts are pale green. (The commoner interloper is found across the Mediterranean basin and is not entitled to the name, which continues in use however. The wrongly named albicans, has brown, papery bracts, and off-white flowers). Our stock was first found near Sana’a, Yemen and is thriving here, outside, in a sunny, raised bed. Iris Sibirica and others Iris chrysographes Black Form Clumps of narrow, iris-like foliage. Tall sprays of darkest violet to almost black velvety flowers, Jun-Sept. Ht 40cm. Moist, well drained soil. Part shade. Deepest Purple which is virtually indistinguishable from black. Moist soil. Ht. 50cm Iris chrysographes Dykes (William Rickatson Dykes, 1911, China); Section Limniris, Series Sibericae; 14-18" (35-45 cm), B7D; Flowers dark reddish violet with gold streaks in the signal area giving it its name (golden writing); Collected by E. H. Wilson in 1908, in China; The Gardeners' Chronicle 49: 362. 1911. The Curtis's Botanical Magazine. tab. 8433 in 1912, gives the following information along with the color illustration. -

Ulster Group Newsletter 2013.Pdf
Newsletter No:12 Contents:- Editorial Obituaries Contributions:- Notes on Lilies Margaret and Henry Taylor Some Iris Species David Ledsham 2nd Czech International Rock Garden Conference Kay McDowell Homage to Catalonia Liam McCaughey Alpine Cuttings - or News Items Show News:- Information:- Web and 'Plant of the Month' Programme 2013 -2014 Editorial After a long cold spring I hope that all our members have been enjoying the beautiful summer, our hottest July for over 100 years. In the garden, flowers, butterflies and bees are revelling in the sunshine and the house martins, nesting in our eaves, are giving flying displays that surpass those of the Red Arrows. There is an emphasis ( almost a fashion) in horticultural circles at the moment on wild life gardening and wild flower meadows. I have always felt that alpines are the wild flowers of the mountains, whether growing in alpine meadows or nestling in among the rocks. Our Society aims to give an appreciation and thus the protection and conservation of wild flowers and plants all over the world. Perhaps you have just picked up this Newsletter and are new to the Society but whether you have a window pot or a few acres you would be very welcome to join the group and find out how much pleasure, in many different ways, these mountain wild flowers can bring. My thanks to our contributors this year who illustrate how varied our interest in plants can be. Not only did the Taylors give us a wonderful lecture and hands-on demonstration last November but kindly followed it up with an article for the Newsletter, and I hope that many of you, like me, have two healthy little pots of lily seedlings thanks to their generous gift of seeds. -

TAXON:Aphananthe Aspera
TAXON: Aphananthe aspera SCORE: 0.0 RATING: Low Risk (Thunb.) Planch. Taxon: Aphananthe aspera (Thunb.) Planch. Family: Cannabaceae Common Name(s): mukutree Synonym(s): Homoioceltis aspera (Thunb.) Blume scabrous aphananthe Prunus aspera Thunb. Assessor: Chuck Chimera Status: Assessor Approved End Date: 25 Jan 2018 WRA Score: 0.0 Designation: L Rating: Low Risk Keywords: Deciduous Tree, Unarmed, Wind-Pollinated, Bird-Dispersed, Coppices Qsn # Question Answer Option Answer 101 Is the species highly domesticated? y=-3, n=0 n 102 Has the species become naturalized where grown? 103 Does the species have weedy races? Species suited to tropical or subtropical climate(s) - If 201 island is primarily wet habitat, then substitute "wet (0-low; 1-intermediate; 2-high) (See Appendix 2) High tropical" for "tropical or subtropical" 202 Quality of climate match data (0-low; 1-intermediate; 2-high) (See Appendix 2) High 203 Broad climate suitability (environmental versatility) y=1, n=0 y Native or naturalized in regions with tropical or 204 y=1, n=0 y subtropical climates Does the species have a history of repeated introductions 205 y=-2, ?=-1, n=0 ? outside its natural range? 301 Naturalized beyond native range y = 1*multiplier (see Appendix 2), n= question 205 n 302 Garden/amenity/disturbance weed n=0, y = 1*multiplier (see Appendix 2) n 303 Agricultural/forestry/horticultural weed n=0, y = 2*multiplier (see Appendix 2) n 304 Environmental weed n=0, y = 2*multiplier (see Appendix 2) n 305 Congeneric weed n=0, y = 1*multiplier (see Appendix 2) n 401 -

Globally Important Agricultural Heritage Systems (GIAHS) Application
Globally Important Agricultural Heritage Systems (GIAHS) Application SUMMARY INFORMATION Name/Title of the Agricultural Heritage System: Osaki Kōdo‟s Traditional Water Management System for Sustainable Paddy Agriculture Requesting Agency: Osaki Region, Miyagi Prefecture (Osaki City, Shikama Town, Kami Town, Wakuya Town, Misato Town (one city, four towns) Requesting Organization: Osaki Region Committee for the Promotion of Globally Important Agricultural Heritage Systems Members of Organization: Osaki City, Shikama Town, Kami Town, Wakuya Town, Misato Town Miyagi Prefecture Furukawa Agricultural Cooperative Association, Kami Yotsuba Agricultural Cooperative Association, Iwadeyama Agricultural Cooperative Association, Midorino Agricultural Cooperative Association, Osaki Region Water Management Council NPO Ecopal Kejonuma, NPO Kabukuri Numakko Club, NPO Society for Shinaimotsugo Conservation , NPO Tambo, Japanese Association for Wild Geese Protection Tohoku University, Miyagi University of Education, Miyagi University, Chuo University Responsible Ministry (for the Government): Ministry of Agriculture, Forestry and Fisheries The geographical coordinates are: North latitude 38°26’18”~38°55’25” and east longitude 140°42’2”~141°7’43” Accessibility of the Site to Capital City of Major Cities ○Prefectural Capital: Sendai City (closest station: JR Sendai Station) ○Access to Prefectural Capital: ・by rail (Tokyo – Sendai) JR Tohoku Super Express (Shinkansen): approximately 2 hours ※Access to requesting area: ・by rail (closest station: JR Furukawa