Using Kodak Chemicals in Minilabs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Film Capture for Digitization
2000 International Symposium on Silver2000 Halide International Technology Symposium on Silver Halide Technology Copyright 2000, IS&T Film Capture for Digitization Allan F. Sowinski, Lois A. Buitano, Steven G. Link, and Gary L. House Imaging Materials and Media, Research & Development Eastman Kodak Company Rochester, NY USA Abstract printing devices, including silver halide paper writers, inkjet printers, and thermal dye transfer printers, that will accept Digital minilab photofinishing is beginning to spread rapidly image data inputs from a variety of sources including digital in the market place, in part, as a means to provide access to still cameras, and film and paper scanners. When film network imaging services, and also to fulfill the printing scanning and digital writing have supplemented traditional needs of the growing base of consumer digital still cameras. optical photofinishing sufficiently, image-taking films When film scanning and digital writing have supplemented designed for optimal scan printing will be feasible. traditional optical photofinishing sufficiently, image-taking Design opportunities to improve silver halide image films designed for optimal scan-printing will be feasible. capture may be afforded as a result of these photofinishing Representative film digitization schemes are surveyed technology changes. Some features of silver halide capture in order to determine some of the optimal features of input may merit improvement or alteration in order for it to silver halide capture media. Key historical features of films remain a very attractive consumer and professional imaging designed for optical printing are considered with respect to technology. In addition, electronic image processing may this new image-printing paradigm. One example of an allow new chemical or emulsion technologies in film system enabled new film feature is recording the scene with design that were difficult to manage with the strict increased color accuracy through theoretically possible requirements of trade optical printing compatibility. -

Requirements for Printing Technologies in Digital Photo Finishing
DPP2001: International Conference on Digital Production Printing and Industrial Applications Requirements for Printing Technologies in Digital Photo Finishing Tobias Damm Agfa Gevaert AG, Consumer Imaging, Lab Equipment D-81539 Munich, Germany Introduction The photo paper is designed to convert the negative film image into the positive photography. The paper Non-impact printing technologies have recently gained a gradation is about γ =2.5 in order to achieve an overall high level of image quality - this allows to produce gradation of the system of about γ =1.4. This results in: photo-like prints. • a high contrast of the prints, In the development of new printing technologies • an improved sharpness of details, and suitable for photo imaging applications, the question of • an enhancement of color saturation. the necessary image quality and the basic parameters affecting this quality, is of extreme importance. It is an advantage specific to the 2 step process – film Our investigation presented here addresses these and paper – that the images can still be improved after questions by defining the minimum technical exposing the film and before printing the photograph. requirements for high quality amateur photo printing. However, there are some shortcomings of the We derive data for visually perceived image quality conventional system which can be corrected when levels using prints produced on a high quality digital processing the images digitally between these two printing system. In this system scan data were generated production steps. from colour negative film, passed through an exactly Generally spoken, the photo paper defines the defined digital processing unit and outputted on a high possible range of parameters of the prints, but the actual quality colour laser recorder. -
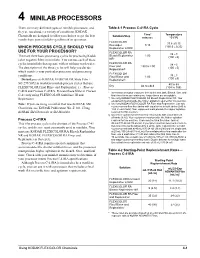
4 Minilab Processors
4 MINILAB PROCESSORS There are many different types of minilab processors, and Table 4-1 Process C-41RA Cycle they are used under a variety of conditions. KODAK Time* Temperature Chemicals are designed to offer you choices to get the best Solution/Step min:sec °C (°F) results from your minilab regardless of its operation. FLEXICOLOR 37.8 ± 0.15 Developer 3:15 (100.0 ± 0.25) WHICH PROCESS CYCLE SHOULD YOU Replenisher LORR USE FOR YOUR PROCESSOR? FLEXICOLOR RA 38 ± 3 Bleach Replenisher 1:00 There are three basic processing cycles for processing Kodak (100 ± 5) color negative films in minilabs. You can use each of these NR† FLEXICOLOR RA cycles in minilabs that operate with or without wash water. 38 ± 3 Fixer and 1:30 to 2:00 The description of the three cycles will help you decide (100 ± 5) Replenisher‡ which matches your particular processor and processing FLEXICOLOR 38 ± 3 conditions. Final Rinse and 1:00 (100 ± 5) Do not process KODAK VERICOLOR Slide Film / Replenisher§ SO-279/5072 in washless minilab process cycles that use 40 to 68 Dry As needed FLEXICOLOR Final Rinse and Replenisher, i.e., Process (104 to 155) C-41B and Process C-41RA. Process these films in Process * Immersion time plus crossover time to the next tank. Bleach, fixer, and C-41 only using FLEXICOLOR Stabilizer III and final rinse times are minimums; longer times are acceptable. Replenisher. † Use only KODAK FLEXICOLOR RA Bleach Replenisher NR. Your equipment must provide the higher agitation required for this solution. Note: If you are using a minilab that uses KODAK SM ‡ Use only KODAK FLEXICOLOR RA Fixer and Replenisher. -

Automatic Image Processing in Digital Photoprinters
IS&T©s 2000 PICS Conference IS&T©s 2000 PICS Conference Copyright 2000, IS&T Automatic Image Processing in Digital Photoprinters Tobias Damm Agfa Gevaert AG, Consumer Imaging, Lab Equipment D-81539 Munich, Germany Abstract In this paper the possibilities to remove shortcomings of the conventional photographic system as well as the expansion The conventional photographic system, based on AgX film of photographic production by digital means, focused on and paper will be briefly described. There are many issues to Minilab and wholesale finishing labs will be treated. In a be improved by digital image enhancement: at under exposed digital system the film is scanned with a high resolution, images the reduced contrast, color saturation and increased high dynamic film scanner. The data are processed and the grain; cross curving of film and paper; the problems due to corrected data are printed by a digital printer. The image files the limited contrast rendering ability of the paper with from digital cameras can also be fed into the processing unit respect to the total dynamic range and the loss of definition to produce prints and digital image files from film scans can at high saturated colors. be delivered. Automatic scratch and dust removal and new products with manipulated color saturation (sepia, black & white) are Conventional Photographic System examples for new possibilities due to digitization of the lab equipment. At the conventional photographic system the color negative The digital workflow is also a prerequisite for the pro- film and paper are well adjusted to each other to provide both duction of prints from digital cameras and to provide digital a good image quality and a high system robustness under files from scanned film. -

FUJICOLOR CRYSTAL ARCHIVE DIGITAL PAPER SUPER TYPE CN Is a Silver Halide Color Paper Designed Exclusively for Digital Output. Wh
1. Features and uses 2. Safelight FUJICOLOR CRYSTAL ARCHIVE DIGITAL PAPER Handle in total darkness. If safelight use is SUPER TYPE CN is a silver halide color paper designed unavoidable, observe the following precautions. exclusively for digital output. When used in conjunction Expose paper no longer than 1 minute to light with medium or large scale digital printer systems or the emitted through two Fuji Safelight Filter No. 103A FUJIFILM FRONTIER DIGITAL MINILAB, this paper (or Wratten Safelight Filter No. 13) in a 10 watt yields high image quality digital prints that make it tungsten lamp safelight located at least 1 meter from suitable for professional and commercial display uses. the work area Safelight filters fade with extended use and need Features regular checking. Replace when paper fogging is detected. High D-max Boasts a wide tonal range, Exposed paper is susceptible to safelight induced producing high image quality prints with a rich sensitivity increases in the exposed area. For this reason, exposed paper should be subjected as textural quality little as possible to safelight illumination. Purer Whiteness Clearer, more distinct print images and sharper text 3. Pre-processing paper handling / storage quality The higher the temperature and humidity, the more Vibrant Color Expanded color paper, whether unused, unexposed or exposed, is Reproduction reproduction range with susceptible to adverse changes in speed, color high color saturation, ideally balance, physical characteristics and other properties. suited to commercial -

User's Guide for KODAK Picture Kiosk Plus Software, V9.X
User's Guide for KODAK Picture Kiosk Plus Software, V9.X Including Information on the: KODAK Picture Kiosk Console 3/4/6/8/9 KODAK Picture Maker G4 Cabinet 17/120 KODAK Picture Maker G4 Cabinet 17/230 KODAK Picture Maker G4 Cabinet 24/120 KODAK Picture Maker G4 Cabinet 24/230 KODAK Picture Maker G4 Cabinet 36/120 KODAK Picture Maker G4 Cabinet 36/230 KODAK MOMENTS M1 Order Station Console 9 Contents Back Next Index Exit Table of Contents 1 Getting Started Showing the Language Selection Overview...................................................... 1-1 Screen........................................................ 2-4 Using This Guide ......................................... 1-2 Using Auto Shutdown and Startup............. 2-5 Before Using the Kiosk ................................ 1-2 Adjusting the Volume ................................. 2-6 Turning on the Kiosk.................................... 1-3 Setting the Time Zone................................ 2-7 Turning off the Kiosk.................................... 1-3 Setting the Date and Time ......................... 2-7 Navigating the Software .............................. 1-4 Setting the Retailer Store Information........ 2-8 Viewing the Shopping Cart .......................... 1-5 Configuring the Receipt Printer Feature .... 2-9 Using the Keyboard and Mouse .................. 1-6 Customizing the Receipt........................ 2-10 Connecting the keyboard and mouse ........ 1-6 Enabling Barcode Printing..................... 2-11 Disconnecting the keyboard and mouse.... 1-6 Sample Receipt ..................................... 2-13 KODAK Picture Kiosk G20 Configuration.... 1-7 Setting up Order Management................. 2-17 KODAK MOMENTS M1 Order Station Setting up the Local Area Network........... 2-18 Configuration ............................................... 1-8 Setting up the Kiosk Network................... 2-20 2 Setting up the Kiosk Sharing Devices with Other Kiosks ....... 2-21 Accessing the Setup Screen ....................... 2-1 Setting up the Host Kiosk ..................... -

R2 Super Series Konica Minolta Digital Minilab System
Konica Minolta supports the digital on-site business For Digital On-Site Business of its customers by providing systems with high connectivity and expandability. Konica Minolta intends to continue working with its customers to develop the future of the digital on-site business. R2 SUPER SERIES KONICA MINOLTA DIGITAL MINILAB SYSTEM R2 SUPER 1000 R2 SUPER 700 R2 SUPER 1000 COMPACT R2 SUPER 700 COMPACT For safest results Carefully read the instruction manual before use and follow directions. Keep away from places subject to water, humidity, dust, oil, steam and other conditions, which may cause fire or other accident. Be sure to use the designated voltage and current. Digital ICE is a trademark of Kodak. Memory Stick is a trademark of Sony Corporation. Windows XP/2000 are registered trademarks of Microsoft Corporation in the United States and elsewhere. Photoshop and Illustrator are registered trademarks of Adobe Corporation in the United States and elsewhere. All other product and company names herein are trademarks of their respective owners. Specifications and design of products may be improved or ortherwise modified without prior notice. KONICA MINOLTA PHOTO IMAGING, INC. No.26-2, Nishishinjuku 1-chome, Shinjuku-ku, Tokyo 163-0512, Japan URL http://konicaminolta.com Ver.3 2004.11 10,000 *AD8 Konica Minolta’s answer for the age of the digital minilab. Performance and features that set the standard. Enabling features standard for your digital on-site business World class 400 x 800 dpi high-resolution image quality. Highly reliable printer, capable of large format prints up to 10 x 15 inches. Minilab system with outstanding network compatibility, enabling the full range of digital services. -

Lucidiom Touch Prints Users Guide
APM Photo Kiosk User’s Guide © 2003-2009 Lucidiom, Inc 8100 Boone Blvd • Suite 310 Vienna, VA 22182 Phone 703.564.3400 • Fax 703.848.3015 i Important Information Login Credentials for www.APMProfitWatcher.com Visit www.apmprofitwatcher.com (formerly www.apmnetwork.com) to view order details, customer information, and usage statistics. Keep track of your retail location’s average order amount and run sales and product reports on a per-kiosk or per-client basis. Username Password APM ID(s) APM ID(s) Activation Lab 50 Credentials Dealer Code Username Password Back Lab Burner ID(s) APM ID(s) Activation ii Table of Contents Important Information ........................................................................................................................................................................... ii Login Credentials for www.APMProfitWatcher.com .................................................................................................................. ii APM ID(s) ............................................................................................................................................................................................... ii Lab 50 Credentials ................................................................................................................................................................................. ii Back Lab Burner ID(s) ......................................................................................................................................................................... -

Photographic Recording of Heritage Items Using Film Or Digital Capture
HERITAGE INFORMATION SERIES PHOTOGRAPHIC RECORDING OF HERITAGE ITEMS USING FILM OR DIGITAL CAPTURE ACKNOWLEDGEMENTS This document was prepared by Lawrie Greenup in 2006 based on the original guidelines, Guidelines for Photographic Recording of Heritage Items produced by Don Godden for the Heritage Office in 1994. DISCLAIMER Any representation, statement, opinion or advice, expressed or implied in this publication is made in good faith but on the basis that the State of New South Wales, its agents and employees are not liable (whether by reason of negligence, lack of care or otherwise) to any person for any damage or loss whatsoever which has occurred or may occur in relation to that person taking or not taking (as the case may be) action in respect of any representation, statement or advice referred to above. Heritage Office Department of Planning Locked Bag 5020 Parramatta NSW 2124 Ph: (02) 9873 8500 Fax: (02) 9873 8599 www.heritage.nsw.gov.au Ó Crown copyright 2006 ISBN 1 921121 02 5 HO 06/03 2 Photographic Recording of Heritage Items INTRODUCTION............................................................................................................5 WH AT YOU NEED TO KNOW ABOUT PHOTOGRAPHIC RECORDING FOR ARCHIVAL PURPOSES ...............................................................................................6 PHOTOGRAPHER'S REQUIREMENTS......................................................................8 EQUIPMENT – FILM-BASED RECORDING................................................................9 CAMERAS .................................................................................................................9 -

Qss-3101 Noritsu
QSS-3101 HIGH PERFORMANCE FULLY DIGITAL MINILAB NORITSU HIGH PERFORMANCE The QSS-3101 Digital is a highly productive minilab, with a superior work flow provided by fully digital operations, and system components that employ state-of-the-art technology. HIGHQUALITYLASER EXPOSURESYSTEM DIGITAL PROCESSING A revolutionary, newly developed laser technology is employed in the exposure Because the QSS-3101 Digital Minilab converts all scanned images into system of the QSS-3101 Digital, enabling high quality prints of 320dpi to be digital data, a wide range of correction measures can be applied to output onto both standard and digital paper. significantly improve contrast, sharpness and red-eye, as well as to minimize problems with exposure, backlighting, and flash photography. Negatives with dust and scratches can be automatically detected and HIGHHIGH CAPACITYCAPACITY corrected when the standard Digital ICE™ feature is employed. From scanning to printing, all operations are fully digital. The utilization of a high-speed, detached scanner, laser print engine, and numerous speed- enhancing features enable the QSS-3101 Digital to produce up to approximately 2,350 5”x3.5” prints per hour. High quality enlargements up to 12”x 18” can be produced as well as other additional value print such as greeting cards, calendar prints, ID photos, business cards and package prints. SIMPLE USER INTERFACE Our easy-to-understand graphic user interface simplifies the printing process so that even operators with limited experience are able to produce a wide range of prints. Icons representing the actual input/output media are displayed on the screen of the QSS-3101 Digital allowing you to check the progress of your orders by simply glancing at the main menu. -

Qss-2801/2802 Noritsu
QSS-2801/2802 HIGH CAPACITY DIGITAL LAB SYSTEMS NORITSU QSS-2801/2802 QSS-2801/2802QSS-2801/2802 digitaldigital • INDUSTRY LEADERS NORITSU AND TEXAS INSTRUMENTS JOIN FORCES TO DEVELOP A HIGH • IMPORT IMAGES FROM VIRTUALLY ANY DIGITAL MEDIUM PERFORMANCE PRINT EXPOSURE ENGINE • OUTPUT HIGH QUALITY PRINTS ON STANDARD SILVER HALIDE PAPER Reflected REVOLUTIONARY NEW DIGITAL PRINTER Image A joint effort between Noritsu and Texas Instruments Light has produced a revolutionary new high performance 135 Negative Film Color Wheel 135 Positive Source digital print engine capable of producing exceptionally Film Greeting Cards high exposure speeds and color saturation. DMD Illumination PRINTS(OUTPUT) DIGITAL Projection Featured in both the QSS-2801 and QSS-2802, the Lens new digital printer utilizes a Digital Micromirror Mirror 135 QSS-2802 120 Negative 120 Positive CD Device™(DMD™) to harness Digital Light Mounted October Positive Film Film Film SUN MON TUE WED THU FRI SAT Processing™(DLP™) technology. This new tech- 12 345 6 7 8 9 10 11 12 Exposure 13 14 15 16 17 18 19 INPUT OUTPUT 20 21 22 23 24 25 26 nology uses digital control to integrate 1.3 million 27 28 29 30 31 Floppy 16-micron-square metal mirrors onto a single chip. Process Direction Calendar Prints The result is a print engine that furnishes unsurpassed IX240 Film Prints 3 DMD (Actual detail and performance when coupled with a high DLP-based printing Micromirrors Top View) speed film scanner and multi-lane paper processing. The operation and structure of DMD IMAGE SOURCES (INPUT) Package Prints Floppy Zip™ CD • FULLY DIGITAL MINILAB SYSTEM OFFERS OPTIONAL LABOR SAVING PERIPHERAL EQUIPMENT Along with the operator friendly design features you’ve come to Digital Camera Index PC Card Flash Card expect from Noritsu, the QSS-2801 and 2802 digital minilabs boast Print a new, user-friendly interface that make system operation a snap. -

Fujicolor Crystal Archive Digital Paper Type Dpii
AF3-212E COLOR NEGATIVE PAPERS FUJICOLOR CRYSTAL ARCHIVE DIGITAL PAPER TYPE DPII 1. FEATURES AND USES 3. PRE-PROCESSING PAPER HANDLING/ STORAGE FUJICOLOR CRYSTAL ARCHIVE DIGTAL PAPER TYPE • The higher the temperature and humidity, the more DPII is a silver halide color paper designed exclusively paper, whether unused, unexposed or exposed, is for digital output. When used in conjunction with susceptible to adverse changes in speed, color bal- medium- or large-scale digital printer systems or the ance, physical characteristics and other properties. FUJI DIGITAL MINILAB FRONTIER, this paper yields Unprocessed paper is best stored at low tempera- high-image-quality digital prints that make it suitable tures. Specifically, the following conditions should be for such professional uses as portrait or commercial used for paper storage. photography. o Short-term storage: Store in a cool and dark Features location, away from direct sunlight, high tem- perature and high humidity ................... Boasts a wide tonal range, • High D-max ° ° producing high-image- o Long-term storage: Below 10 C (50 F) quality prints with a rich • Raw paper which has been stored at a low textural quality temperature (by refrigeration) should be set aside • Purer Whiteness ........... Clearer, more distinct print and allowed to warm to room temperature prior to images and sharper text being opened. If the paper is taken out of its quality packaging immediately after being removed from • Vibrant Color ................. Expanded color reproduction refrigerated storage, condensation will form on the Reproduction range with high color paper surfaces, resulting in print color changes and saturation, ideally suited to easily damaged surfaces.