PTERIDOLOGIST 2017 Contents: Volume 6 Part 4, 2017
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Annual Report 2018
ANNUAL REPORT 2018 Penicuik CAB 2 Service Provision Main office Opening Hours Palmer House 14 & 14a John Street Monday to Thursday Penicuik 9:30 to 3:30 EH26 8AB Friday 9:30 to 1:30 Advice & appointments 01968 675259 Freephone Advice Line 0800 0327077 Money Advice 01968 679918 Outreach clinics Loanhead Library Monday: 10:00 to 1:00 MAEDT Mayfield Monday 10: to 1:00 Lasswade Library Tuesdays 9:30 to 11:30 Loganlea Centre Tuesday 1:30 to 3:00 by appointment Loanhead Miners Welfare Wednesday: 10:00 to 1:00 VOCAL Dalkeith By appointment through VOCAL. Midlothian Sure Start Centres Tuesday, Wednesday and Thursday . Home visits to the housebound By appointment Registered Charity no: SC014421. Company Limited by guarentee no: SC229838 3 TABLE OF CONTENTS Service provision 2 4 Chairperson's report Penicuik CAB Who we are 5 What we do 5 What we did 6-7 8 Funded Projects Benefit Advice for Carers 8 Kinship Care . AIM HI - Advice for families Finacial Reports Financial Summary 10 Financial 11 Statements Statement of Director's 12 Responsibilites Independent Examiner's Report 13-14 Statement of Finacial Activity 15 Balance sheet 16 Notes to Financial statements 17-22 Staffing 23 4 CHAIRPERSON’S REPORT The unassuming stone façade of 14A John Street, Penicuik, belies the good work that takes place within. Every day, under the caring and dedicated management of Sue Peart and Russell Gray, the staff and volunteers have been helping the many clients who seek their advice. Most advice is about housing, employment, debt and of course, universal credit. -

Lista Anotada De La Taxonomía Supraespecífica De Helechos De Guatemala Elaborada Por Jorge Jiménez
Documento suplementario Lista anotada de la taxonomía supraespecífica de helechos de Guatemala Elaborada por Jorge Jiménez. Junio de 2019. [email protected] Clase Equisetopsida C. Agardh α.. Subclase Equisetidae Warm. I. Órden Equisetales DC. ex Bercht. & J. Presl a. Familia Equisetaceae Michx. ex DC. 1. Equisetum L., tres especies, dos híbridos. β.. Subclase Ophioglossidae Klinge II. Órden Psilotales Prantl b. Familia Psilotaceae J.W. Griff. & Henfr. 2. Psilotum Sw., dos especies. III. Órden Ophioglossales Link c. Familia Ophioglossaceae Martinov c1. Subfamilia Ophioglossoideae C. Presl 3. Cheiroglossa C. Presl, una especie. 4. Ophioglossum L., cuatro especies. c2. Subfamilia Botrychioideae C. Presl 5. Botrychium Sw., tres especies. 6. Botrypus Michx., una especie. γ. Subclase Marattiidae Klinge IV. Órden Marattiales Link d. Familia Marattiaceae Kaulf. 7. Danaea Sm., tres especies. 8. Marattia Sw., cuatro especies. δ. Subclase Polypodiidae Cronquist, Takht. & W. Zimm. V. Órden Osmundales Link e. Familia Osmundaceae Martinov 9. Osmunda L., una especie. 10. Osmundastrum C. Presl, una especie. VI. Órden Hymenophyllales A.B. Frank f. Familia Hymenophyllaceae Mart. f1. Subfamilia Trichomanoideae C. Presl 11. Abrodictyum C. Presl, una especie. 12. Didymoglossum Desv., nueve especies. 13. Polyphlebium Copel., cuatro especies. 14. Trichomanes L., nueve especies. 15. Vandenboschia Copel., tres especies. f2. Subfamilia Hymenophylloideae Burnett 16. Hymenophyllum Sm., 23 especies. VII. Órden Gleicheniales Schimp. g. Familia Gleicheniaceae C. Presl 17. Dicranopteris Bernh., una especie. 18. Diplopterygium (Diels) Nakai, una especie. 19. Gleichenella Ching, una especie. 20. Sticherus C. Presl, cuatro especies. VIII. Órden Schizaeales Schimp. h. Familia Lygodiaceae M. Roem. 21. Lygodium Sw., tres especies. i. Familia Schizaeaceae Kaulf. 22. -

Download Document
African countries and neighbouring islands covered by the Synopsis. S T R E L I T Z I A 23 Synopsis of the Lycopodiophyta and Pteridophyta of Africa, Madagascar and neighbouring islands by J.P. Roux Pretoria 2009 S T R E L I T Z I A This series has replaced Memoirs of the Botanical Survey of South Africa and Annals of the Kirstenbosch Botanic Gardens which SANBI inherited from its predecessor organisations. The plant genus Strelitzia occurs naturally in the eastern parts of southern Africa. It comprises three arborescent species, known as wild bananas, and two acaulescent species, known as crane flowers or bird-of-paradise flowers. The logo of the South African National Biodiversity Institute is based on the striking inflorescence of Strelitzia reginae, a native of the Eastern Cape and KwaZulu-Natal that has become a garden favourite worldwide. It sym- bolises the commitment of the Institute to champion the exploration, conservation, sustain- able use, appreciation and enjoyment of South Africa’s exceptionally rich biodiversity for all people. J.P. Roux South African National Biodiversity Institute, Compton Herbarium, Cape Town SCIENTIFIC EDITOR: Gerrit Germishuizen TECHNICAL EDITOR: Emsie du Plessis DESIGN & LAYOUT: Elizma Fouché COVER DESIGN: Elizma Fouché, incorporating Blechnum palmiforme on Gough Island PHOTOGRAPHS J.P. Roux Citing this publication ROUX, J.P. 2009. Synopsis of the Lycopodiophyta and Pteridophyta of Africa, Madagascar and neighbouring islands. Strelitzia 23. South African National Biodiversity Institute, Pretoria. ISBN: 978-1-919976-48-8 © Published by: South African National Biodiversity Institute. Obtainable from: SANBI Bookshop, Private Bag X101, Pretoria, 0001 South Africa. -

NEWSLETTER December 2017 Previous Issue: September 2017 ISSN 1171-9982
NEWSLETTER December 2017 Previous issue: September 2017 ISSN 1171-9982 From the President Articles for web site The October WBS meeting gave me much hope for the future of botanical We welcome articles for research in New Zealand. We were lucky to hear talks from two of our 2016 consideration for inclusion on WBS prizewinners. Jubilee Award winner, Stacey Bryan, on behalf of Hannah our web site: Buckley, gave a fascinating talk on pīngao, which wove in aspects of genetics, www.wellingtonbotsoc.org.nz culture, conservation and ecology. Grants to Graduate Students prizewinner, Nathaniel Walker-Hale, gave a very polished presentation on the evolution of salt Please send your article to: tolerance and betalain pigments. Nathaniel was recently awarded a Woolf Fisher Richard Herbert Scholarship to continue his studies with a PhD at the University of Cambridge e-mail [email protected] in the UK. Lastly, Jane Humble gave an insightful talk into botanical art and brought along some of her own artworks for us to admire. The talks stimulated Writing for the Bulletin much discussion. I had several members tell me how much they enjoyed the Do you have a botanical observation, evening in the days afterwards. Thanks to all our speakers, and to Sunita Singh anecdote, or insight that you could for organising our meeting programme. share with others in BotSoc? If so, Lara Shepherd, President please consider contributing it to the Wellington Botanical Society New members Bulletin. There is still plenty of space We welcome the following: in the next issue. For more details and Barbara Hammonds, Tom Mayo, Sarah Wilcox, Joyce Wilson. -

The Story of Creag Meagaidh National Nature Reserve
Scotland’s National Nature Reserves For more information about Creag Meagaidh National Nature Reserve please contact: Scottish Natural Heritage, Creag Meagaidh NNR, Aberarder, Kinlochlaggan, Newtonmore, Inverness-shire, PH20 1BX Telephone/Fax: 01528 544 265 Email: [email protected] The Story of Creag Meagaidh National Nature Reserve The Story of Creag Meagaidh National Nature Reserve Foreword Creag Meagaidh National Nature Reserve (NNR), named after the great whalebacked ridge which dominates the Reserve, is one of the most diverse and important upland sites in Scotland. Creag Meagaidh is a complex massif, with numerous mountain tops and an extensive high summit plateau edged by a dramatic series of ice-carved corries and gullies. The Reserve extends from the highest of the mountain tops to the shores of Loch Laggan. The plateau is carpeted in moss-heath and is an important breeding ground for dotterel. The corries support unusual artic- alpine plants and the lower slopes have scattered patches of ancient woodland dominated by birch. Located 45 kilometres (km) northeast of Fort William and covering nearly 4,000 hectares (ha), the Reserve is owned and managed by Scottish Natural Heritage (SNH). Creag Meagaidh has been a NNR since 1986 and during the last twenty years SNH has worked to restore natural habitats, particularly woodland, on the Reserve. Like much of the Highlands, the vegetation has been heavily grazed for centuries, so it was decided to reduce the number of grazing animals by removing sheep and culling red deer. The aim was not to eliminate grazing animals altogether, but to keep numbers at a level that allowed the habitats, especially the woodland, to recover. -

Fern Gazette
FERN GAZETTE I INDEX! VOLUME 12 FERN GAZETTE - INDEX VOLUME 12 Aconiopteris 342 Arthromeris wallichiana 87 Acrophorus 315, 317, 318 Aspidium 247 Acropterygium 213 goeringianum 246 Acrostichum 185 sagenioides 315, 316 aureum 98 trifoliatum 318 speciosum 98 Asplenium 50,74, 80, 102, Actinostachys digitate 366 106, 115, 188,189, Acystopteris 304, 313 246, 286,287, 290, Adenophorus 340 304,308,327,331 Adiantopsis 327 adiantum-nigrum 5-8,16,17, 22, 24, radiate 323 78,80, 103-106, Adiantum 31, 36, 78, 215, 115, 116,136, 137' 221, 355, 365 143,144, 149, 152, capillus-veneris 11 ' 12, 1 5, 1 6, 252,255,259,306,363 23-25, 78, 81, aethiopicum 50 84, 151' 195, 263, x alternifolium 309 264, 309, 355, 359 anceps 157, 158 caudatum 50 bil/etii 214 edgeworthii 84 billotii 17, 116, 264, 331, incisum 85 332 latifo/ium 98 bourgaei 271-274 lunulatum 85 breynii 309 malesianum 211 bulbiferum 33 peruvianum 355-359 calcico/a 214 pseudotinctum 323, 326-328 ceterach 18, 19, 23;-25, raddianum 195 133, 136, 144, reniforme 156 152, 252, 255, stenochlamys 360 259, 302, 306, tetraphyllum 323 363 venustum 85 claussenii 324, 327, 328 Africa, Macrothe/ypteris new to, 117 coenobiale 214 Aglaomorpha 225-228 x confluens 252, 259, 301' ·pi/osa 227 302, 362, 363 Aleuritopteris 85 cunelfolium 5-8, 1 03-106 Alsophila 287 dalhousiae 87 bryophila 287 ensiforme 87 dregei 195 exiguum 87 dryopteroides 287 fissum 81 Amauropelta 160 fontanum 81, 271, 331 Ampe/opteris prolifera 87 foreziense 331 Amphineuron opulentum 98 fuscipes 214 Ant1mia 327,328 gemmiferum 193 dregeana 193 g/andulosum -

Insights on Reticulate Evolution in Ferns, with Special Emphasis on the Genus Ceratopteris
Utah State University DigitalCommons@USU All Graduate Theses and Dissertations Graduate Studies 8-2021 Insights on Reticulate Evolution in Ferns, with Special Emphasis on the Genus Ceratopteris Sylvia P. Kinosian Utah State University Follow this and additional works at: https://digitalcommons.usu.edu/etd Part of the Ecology and Evolutionary Biology Commons Recommended Citation Kinosian, Sylvia P., "Insights on Reticulate Evolution in Ferns, with Special Emphasis on the Genus Ceratopteris" (2021). All Graduate Theses and Dissertations. 8159. https://digitalcommons.usu.edu/etd/8159 This Dissertation is brought to you for free and open access by the Graduate Studies at DigitalCommons@USU. It has been accepted for inclusion in All Graduate Theses and Dissertations by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. INSIGHTS ON RETICULATE EVOLUTION IN FERNS, WITH SPECIAL EMPHASIS ON THE GENUS CERATOPTERIS by Sylvia P. Kinosian A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in Ecology Approved: Zachariah Gompert, Ph.D. Paul G. Wolf, Ph.D. Major Professor Committee Member William D. Pearse, Ph.D. Karen Mock, Ph.D Committee Member Committee Member Karen Kaphiem, Ph.D Michael Sundue, Ph.D. Committee Member Committee Member D. Richard Cutler, Ph.D. Interim Vice Provost of Graduate Studies UTAH STATE UNIVERSITY Logan, Utah 2021 ii Copyright © Sylvia P. Kinosian 2021 All Rights Reserved iii ABSTRACT Insights on reticulate evolution in ferns, with special emphasis on the genus Ceratopteris by Sylvia P. Kinosian, Doctor of Philosophy Utah State University, 2021 Major Professor: Zachariah Gompert, Ph.D. -

USAMVB Timisoara 27 November 2020
USAMVB Timisoara ”YOUNG PEOPLE AND MULTIDISCIPLINARY RESEARCH ”Young people and multidisciplinary IN APPLIED LIFE SCIENCES” research in applied life sciences” 27 November 2020 ASTER GENUS IN “ALEXANDRU BELDIE” HERBARIUM FROM “MARIN DRĂCEA” NATIONAL INSTITUTE FOR RESEARCH AND DEVELOPMENT IN FORESTRY CIONTU C. I.1*, DINCĂ MARIA2 1“Marin Drăcea” National Institute for Research and Development in Forestry, Timișoara, Romania 2 “Marin Drăcea” National Institute for Research and Development in Forestry, Brașov, Romania Abstract: Aster Genus is well represented within Alexandru Beldie Herbarium from „Marin Drăcea” National Institute for Research and Development in Forestry. This aspect is proved by a significant number that amounts to 125 vouchers that contain plants from this genus as well as by the information contained in them. These refer to the plants’ harvesting places which cover the entire country, as well as to renowned specialists who have contributed to the collection’s development by harvesting or identifying Aster plants. The present paper organizes and presents species from Aster genus present in the above-mentioned herbarium, amounting to 36 species in 125 vouchers. The species were analysed based on their harvesting place and year, as well as on the specialist who gathered them. Additional criteria are also present such as: drawer’s number, voucher’s number, botanic collection, specie’s name, harvesting date, harvesting place, the specialist who has collected and / or determined the species, and the conservation degree. The herbarium hosts three Aster samples that belong to a species present in the Red Book of superior Romanian plants (Aster canus W. et. K.). Furthermore, the herbarium can take pride in old plants, with an historical value, that were collected 170 years ago (Aster tripolium L.,1849, Aster amellus L., 1851). -
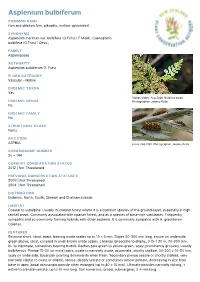
Asplenium Bulbiferum
Asplenium bulbiferum COMMON NAME Hen and chicken fern, pikopiko, mother spleenwort SYNONYMS Asplenium marinum var. bulbifera (G.Forst.) F.Muell.; Caenopteris bulbifera (G.Forst.) Desv.; FAMILY Aspleniaceae AUTHORITY Asplenium bulbiferum G. Forst. FLORA CATEGORY Vascular – Native ENDEMIC TAXON Yes Stokes Valley. Aug 2006. Bulbil on bulbil. ENDEMIC GENUS Photographer: Jeremy Rolfe No ENDEMIC FAMILY No STRUCTURAL CLASS Ferns NVS CODE ASPBUL sorus. Feb 1981. Photographer: Jeremy Rolfe CHROMOSOME NUMBER 2n = 144 CURRENT CONSERVATION STATUS 2012 | Not Threatened PREVIOUS CONSERVATION STATUSES 2009 | Not Threatened 2004 | Not Threatened DISTRIBUTION Endemic. North, South, Stewart and Chatham Islands HABITAT Coastal to subalpine. Usually in lowland forest where it is a common species of the ground-layer, especially in high rainfall areas. Commonly associated with riparian forest, and as a species of base-rich substrates. Frequently sympatric and so commonly forming hybrids with other asplenia. It is commonly sympatric with A. gracillimum Colenso. FEATURES Rhizome short, stout, erect, bearing ovate scales up to 15 × 5 mm. Stipes 50-300 mm long, brown on underside, green above, stout, covered in small brown ovate scales. Laminae lanceolate to elliptic, 0.15-1.20 m, 70-300 mm, bi- to tripinnate, sometimes bearing bulbils. Raches pale green to yellow-green, scaly, prominently grooved, usually bulbiferous. Pinnae 15-30 (or more) pairs, ovate to narrowly ovate, acuminate, shortly stalked, 30-200 × 10-50 mm, scaly on underside, basal pair pointing downwards when fresh. Secondary pinnae sessile or shortly stalked, very narrowly elliptic to ovate or elliptic, obtuse, deeply serrate or sometimes almost pinnate, decreasing in size from base to apex, basal acroscopic pinnule often enlarged (up to 40 × 10 mm). -

Fern Gazette Vol 18 Part 1 V7.Qxd
FERN GAZ. 18(5):264-282. 2009 264 DESICCATION TOLERANCE IN SOME BRITISH FERNS M.C.F. PROCTOR School of Biosciences, University of Exeter, Geoffrey Pope Building, Stocker Road, Exeter EX4 4QD Key-words: Asplenium, chlorophyll fluorescence, drying rate, light responses, Polypodium, recovery rate, relative humidity, relative water content. ABSTRACT Leaves of ten British fern species were tested for their tolerance of desiccation. Asplenium ruta-muraria, A. septentrionale, A. trichomanes, A. ceterach, Polypodium cambricum and P. interjectum withstood drying for periods of a week or more to a relative water content (RWC) of c. 4–7%. This is far below the RWC (c. 30%) at which most vascular-plant tissues are irretrievably damaged. One population of Asplenium adiantum-nigrum was desiccation tolerant, another was not. Aspelnium obovatum was fairly tolerant, behaviour differing with intensity of desiccation and in old and young growth. Polypodium cambricum and P interjectum were both highly tolerant. Polystichum aculeatum was not tolerant. Recovery rates of RWC and the chlorophyll-fluorescence parameter Fv/Fm did not vary greatly between species, with half-recovery times around 2–4 h. The small Asplenium species and A. ceterach dried quickly (half- drying times a few hours), suggesting little stomatal control over drying. The much slower drying of the Polypodium species suggests that their stomata close under water stress. Photosynthetic electron flow in most species saturated at a quarter to a half of full summer sunlight. Asplenium ruta-muraria, A. septentrionale and A. trichomanes showed a similar tendency to non-saturating electron flow at high irradiances as many desiccation-tolerant bryophytes. -

2011 Biodiversity Snapshot. Guernsey Appendices
UK Overseas Territories and Crown Dependencies: 2011 Biodiversity snapshot. Guernsey: Appendices. Author: Dr Charles David Guernsey Biological Records Centre, States of Guernsey Environment Department & La Societe Guernesiaise. More information available at: www.biologicalrecordscentre.gov.gg This section includes a series of appendices that provide additional information relating to that provided in the Guernsey chapter of the publication: UK Overseas Territories and Crown Dependencies: 2011 Biodiversity snapshot. All information relating to Guernsey is available at http://jncc.defra.gov.uk/page-5743 The entire publication is available for download at http://jncc.defra.gov.uk/page-5821 Commissioned by the States of Guernsey Environment Department for the Joint Nature Conservation Committee Prepared by Dr C T David Guernsey Biological Records Centre August 2010 1 Contents Appendix 1: Bailiwick of Guernsey – Location and Introduction ............................. 3 Location, Area, Number of Islands, Population 3 Topography 4 Main economic sectors 4 Constitutional Position 4 Appendix 2: Multilateral Environmental Agreements. ............................................... 5 Appendix 3: National Legislation ................................................................................ 8 Planning 8 Ancient Monuments 8 Coast and beaches 8 Land 8 Fauna 8 Flora 9 Trees 9 Import/export 9 Marine environment 9 Waste 9 Water 9 Appendix 4: National Strategies ................................................................................ 11 Appendix -

TAXONOMY Family Names Scientific Names GENERAL INFORMATION
Plant Propagation Protocol for Athyrium americanum ESRM 412 – Native Plant Production TAXONOMY Family Names Family Scientific Name: Dryopteridaceae (USDA) Family Common Name: Wood fern family (USDA) Scientific Names Genus: Athyrium Roth Species: Athyrium americanum (Butters) Maxon Species Authority: (Butters) Maxon Common Synonym(s) • Athyrium alpestre (Hoppe) Milde (include full scientific • Athyrium alpestre (Hoppe) Milde names (e.g., Elymus ssp. americanum (Butters) Lellinger glaucus Buckley), • Athyrium alpestre (Hoppe) Milde including variety or var. americanum Butters subspecies information) • Athyrium alpestre (Hoppe) Milde var. gaspense Fernald • Athyrium distentifolium Tausch ex Opiz ssp. americanum (Butters) Hultén • Athyrium distentifolium Tausch ex Opiz var. americanum (Butters) B. Boivin Common Name(s): Alpine ladyfern (USDA) Species Code (as per USDA ATAM Plants database): GENERAL INFORMATION Geographical range (distribution maps for North America and Washington state) green=present white=absent Ecological distribution A. americanum is a circumboreal species, occurring at, often (ecosystems it occurs in, near timberline, in rocky slopes and stream borders (Wick). etc): Climate and elevation range Mid to high montane elevations (Wick). Local habitat and Moist acidic soil among rocks, meadows and talus slopes from abundance; may include mid to high elevations often near timberline (Ellingboe). commonly associated species Plant strategy type / - successional stage (stress- tolerator, competitor, weedy/colonizer, seral, late successional) Plant characteristics (life • Forb/herb form (shrub, grass, forb), • Perennial (USDA). longevity, key characteristics, etc) PROPAGATION DETAILS Ecotype (this is meant - primarily for experimentally derived protocols, and is a description of where the seed that was tested came from): Propagation Goal (Options: Plants (Wick). Plants, Cuttings, Seeds, Bulbs, Somatic Embryos, and/or Other Propagules): Propagation Method Seed (Wick).