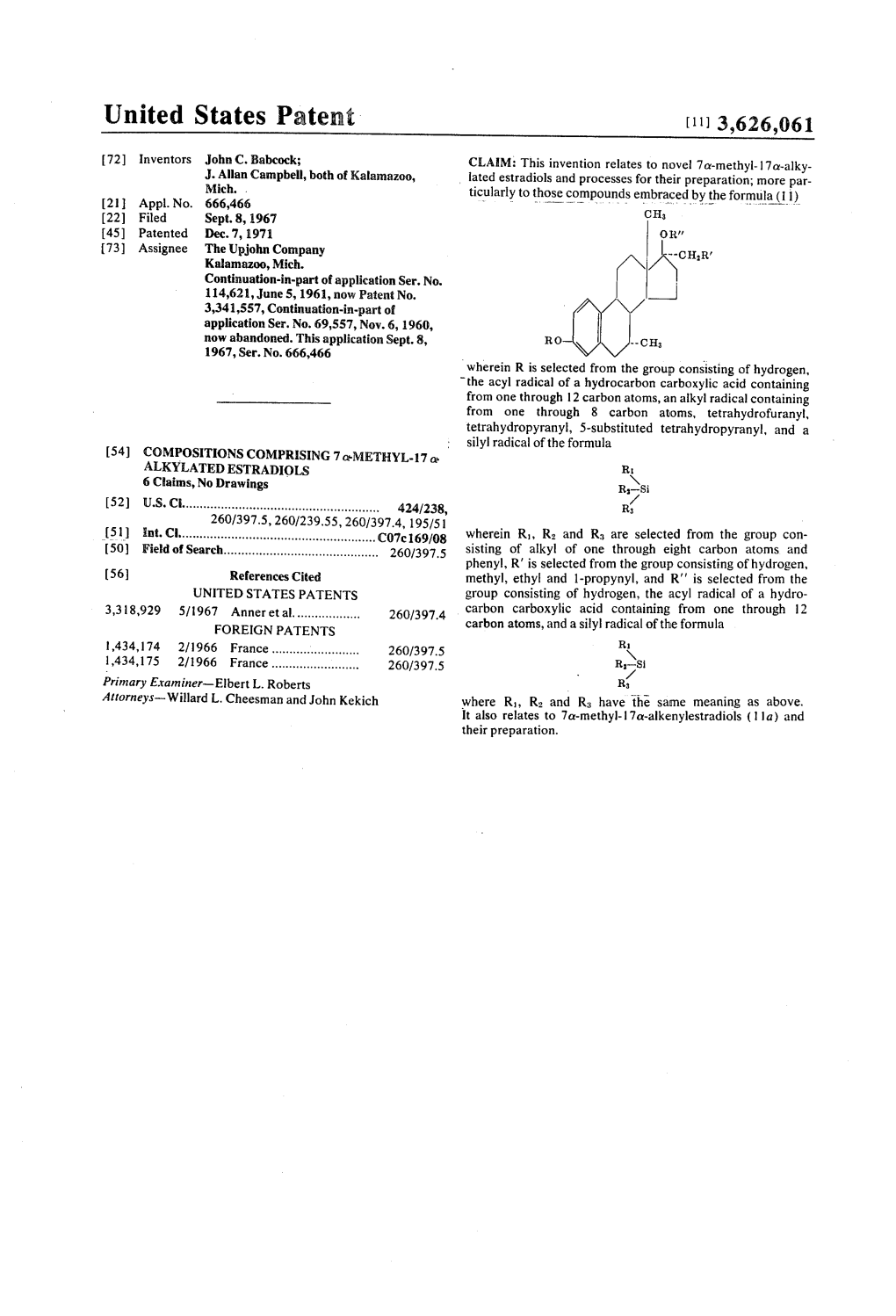
United States Patent [11] 3,626,061 72) Inventors John C
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Critical Role of Oxidative Stress in Estrogen-Induced Carcinogenesis
Critical role of oxidative stress in estrogen-induced carcinogenesis Hari K. Bhat*†, Gloria Calaf‡, Tom K. Hei*‡, Theresa Loya§, and Jaydutt V. Vadgama¶ *Department of Environmental Health Sciences, Mailman School of Public Health, 60 Haven Avenue-B1, Columbia University, New York, NY 10032; ‡Center for Radiological Research, Columbia University, New York, NY 10032; and Departments of §Pathology and ¶Medicine, Charles Drew University, Los Angeles, CA 90059 Communicated by Donald C. Malins, Pacific Northwest Research Institute, Seattle, WA, December 27, 2002 (received for review August 22, 2002) Mechanisms of estrogen-induced tumorigenesis in the target quinones generates oxidative stress and potentially harmful free organ are not well understood. It has been suggested that oxida- radicals that are postulated to be required for the carcinogenic tive stress resulting from metabolic activation of carcinogenic process, and analogous to the metabolic activation of hydrocar- estrogens plays a critical role in estrogen-induced carcinogenesis. bons and other nonsteroidal estrogen carcinogens (9, 19–22). We We tested this hypothesis by using an estrogen-induced hamster have investigated the role of oxidative stress in estrogen carci- renal tumor model, a well established animal model of hormonal nogenesis by using a well established hamster renal tumor model carcinogenesis. Hamsters were implanted with 17-estradiol (E2), that shares several characteristics with human breast and uterine 17␣-estradiol (␣E2), 17␣-ethinylestradiol (␣EE), menadione, a com- cancers, pointing to a common mechanistic origin (6, 9, 23). bination of ␣E2 and ␣EE, or a combination of ␣EE and menadione Different estrogens used in the present study differ in their for 7 months. -

Schwangerschaften Während Der Anwendung Kontrazeptiver Methoden
Journal für Reproduktionsmedizin und Endokrinologie – Journal of Reproductive Medicine and Endocrinology – Andrologie • Embryologie & Biologie • Endokrinologie • Ethik & Recht • Genetik Gynäkologie • Kontrazeption • Psychosomatik • Reproduktionsmedizin • Urologie Schwangerschaften während der Anwendung kontrazeptiver Methoden - Eine Stellungnahme der Deutschen Gesellschaft für Gynäkologische Endokrinologie und Fortpflanzungsmedizin (DGGEF) e.V. und des Berufsverbands der Frauenärzte (BVF) e.V. Rabe T, Goeckenjan M, Dikow N, Schaefer C, Ahrendt HJ Mueck AO, Merkle E, Merki G, Egarter C, König K Albring C J. Reproduktionsmed. Endokrinol 2013; 10 (4), 214-234 www.kup.at/repromedizin Online-Datenbank mit Autoren- und Stichwortsuche Offizielles Organ: AGRBM, BRZ, DVR, DGA, DGGEF, DGRM, D·I·R, EFA, OEGRM, SRBM/DGE Indexed in EMBASE/Excerpta Medica/Scopus Krause & Pachernegg GmbH, Verlag für Medizin und Wirtschaft, A-3003 Gablitz FERRING-Symposium digitaler DVR 2021 Mission possible – personalisierte Medizin in der Reproduktionsmedizin Was kann die personalisierte Kinderwunschbehandlung in der Praxis leisten? Freuen Sie sich auf eine spannende Diskussion auf Basis aktueller Studiendaten. SAVE THE DATE 02.10.2021 Programm 12.30 – 13.20Uhr Chair: Prof. Dr. med. univ. Georg Griesinger, M.Sc. 12:30 Begrüßung Prof. Dr. med. univ. Georg Griesinger, M.Sc. & Dr. Thomas Leiers 12:35 Sind Sie bereit für die nächste Generation rFSH? Im Gespräch Prof. Dr. med. univ. Georg Griesinger, Dr. med. David S. Sauer, Dr. med. Annette Bachmann 13:05 Die smarte Erfolgsformel: Value Based Healthcare Bianca Koens 13:15 Verleihung Frederik Paulsen Preis 2021 Wir freuen uns auf Sie! Schwangerschaften während der Anwendung kontrazeptiver Methoden Schwangerschaften während der Anwendung kontrazeptiver Methoden* Eine Stellungnahme der Deutschen Gesellschaft für Gynäkologische Endokrinologie und Fortpflanzungsmedizin (DGGEF) e.V. -

Pp375-430-Annex 1.Qxd
ANNEX 1 CHEMICAL AND PHYSICAL DATA ON COMPOUNDS USED IN COMBINED ESTROGEN–PROGESTOGEN CONTRACEPTIVES AND HORMONAL MENOPAUSAL THERAPY Annex 1 describes the chemical and physical data, technical products, trends in produc- tion by region and uses of estrogens and progestogens in combined estrogen–progestogen contraceptives and hormonal menopausal therapy. Estrogens and progestogens are listed separately in alphabetical order. Trade names for these compounds alone and in combination are given in Annexes 2–4. Sales are listed according to the regions designated by WHO. These are: Africa: Algeria, Angola, Benin, Botswana, Burkina Faso, Burundi, Cameroon, Cape Verde, Central African Republic, Chad, Comoros, Congo, Côte d'Ivoire, Democratic Republic of the Congo, Equatorial Guinea, Eritrea, Ethiopia, Gabon, Gambia, Ghana, Guinea, Guinea-Bissau, Kenya, Lesotho, Liberia, Madagascar, Malawi, Mali, Mauritania, Mauritius, Mozambique, Namibia, Niger, Nigeria, Rwanda, Sao Tome and Principe, Senegal, Seychelles, Sierra Leone, South Africa, Swaziland, Togo, Uganda, United Republic of Tanzania, Zambia and Zimbabwe America (North): Canada, Central America (Antigua and Barbuda, Bahamas, Barbados, Belize, Costa Rica, Cuba, Dominica, El Salvador, Grenada, Guatemala, Haiti, Honduras, Jamaica, Mexico, Nicaragua, Panama, Puerto Rico, Saint Kitts and Nevis, Saint Lucia, Saint Vincent and the Grenadines, Suriname, Trinidad and Tobago), United States of America America (South): Argentina, Bolivia, Brazil, Chile, Colombia, Dominican Republic, Ecuador, Guyana, Paraguay, -

U.S. Medical Eligibility Criteria for Contraceptive Use, 2010
Morbidity and Mortality Weekly Report www.cdc.gov/mmwr Early Release May 28, 2010 / Vol. 59 U.S. Medical Eligibility Criteria for Contraceptive Use, 2010 Adapted from the World Health Organization Medical Eligibility Criteria for Contraceptive Use, 4th edition department of health and human services Centers for Disease Control and Prevention Early Release CONTENTS The MMWR series of publications is published by the Office of Surveillance, Epidemiology, and Laboratory Services, Centers for Introduction .............................................................................. 1 Disease Control and Prevention (CDC), U.S. Department of Health Methods ................................................................................... 2 and Human Services, Atlanta, GA 30333. How to Use This Document ......................................................... 3 Suggested Citation: Centers for Disease Control and Prevention. [Title]. MMWR Early Release 2010;59[Date]:[inclusive page numbers]. Using the Categories in Practice ............................................... 3 Recommendations for Use of Contraceptive Methods ................. 4 Centers for Disease Control and Prevention Contraceptive Method Choice .................................................. 4 Thomas R. Frieden, MD, MPH Director Contraceptive Method Effectiveness .......................................... 4 Peter A. Briss, MD, MPH Unintended Pregnancy and Increased Health Risk ..................... 4 Acting Associate Director for Science Keeping Guidance Up to Date ................................................... -

Steroids – Basic Science
STEROIDS – BASIC SCIENCE Edited by Hassan Abduljabbar Steroids – Basic Science Edited by Hassan Abduljabbar Published by InTech Janeza Trdine 9, 51000 Rijeka, Croatia Copyright © 2011 InTech All chapters are Open Access distributed under the Creative Commons Attribution 3.0 license, which allows users to download, copy and build upon published articles even for commercial purposes, as long as the author and publisher are properly credited, which ensures maximum dissemination and a wider impact of our publications. After this work has been published by InTech, authors have the right to republish it, in whole or part, in any publication of which they are the author, and to make other personal use of the work. Any republication, referencing or personal use of the work must explicitly identify the original source. As for readers, this license allows users to download, copy and build upon published chapters even for commercial purposes, as long as the author and publisher are properly credited, which ensures maximum dissemination and a wider impact of our publications. Notice Statements and opinions expressed in the chapters are these of the individual contributors and not necessarily those of the editors or publisher. No responsibility is accepted for the accuracy of information contained in the published chapters. The publisher assumes no responsibility for any damage or injury to persons or property arising out of the use of any materials, instructions, methods or ideas contained in the book. Publishing Process Manager Bojan Rafaj Technical Editor Teodora Smiljanic Cover Designer InTech Design Team Image Copyright loriklaszlo, 2011. DepositPhotos First published December, 2011 Printed in Croatia A free online edition of this book is available at www.intechopen.com Additional hard copies can be obtained from [email protected] Steroids – Basic Science, Edited by Hassan Abduljabbar p. -

Endocrine Dynamic Function Protocols
South Eastern Area Laboratory Services DEPARTMENT OF CLINICAL CHEMISTRY & ENDOCRINOLOGY SEALS Pathology Service Randwick Campus Dynamic Function Protocol DETAILS OF TESTS AVAILABLE AND SPECIMENS REQUIRED Compiled by: Phoebe Stanford Registrar Dept of Clinical Chemistry and Endocrinology SEALS North South Eastern Area Laboratory Services SEALS PATHOLOGY SERVICE RANDWICK CAMPUS TABLE OF CONTENTS PATIENT PREPARATION……………………………………………………….. 4 PROCEDURE FOR USING THE GLUCOMETER…………………………….. 6 PANCREAS DIABETES GLUCOSE TOLERANCE TEST …………………………………………………………………………..7 GESTATIONAL DIABETES SCREENING………………………………………………………………..8 GTT FOR INSULIN RESISTANCE (PCOS)…………………………………………………………….10 MEAL TOLERANCE……………………………………………………………………………………….10 EXTENDED GLUCOSE TOLERANCE TEST……………………………………………………11 ANTERIOR PITUITARY ANTERIOR PITUITARY FUNCTION ITT………………………………………………………………………………………………..12 CUSHING'S DISEASE OVERNIGHT DEXAMETHASONE TEST……………………………………………………15 BILATERAL SIMULTANEOUS INFERIOR PETROSAL SINUS SAMPLING…………….16 ACROMEGALY GROWTH HORMONE SUPPRESSION TEST (OGTT) FOR ACROMEGALY……………26 POSTERIOR PITUITARY DIABETES INSIPIDUS WATER DEPRIVATION TEST………………………………………………………………..27 ADRENAL ADRENAL INSUFFICIENCY SHORT SYNACTHEN TEST…………………………………………………………………..30 SYNACTHEN TEST - CONGENITAL ADRENAL HYERPLASIA………………………….32 HYPERALDOSTERONISM SALINE SUPPRESSION TEST………………………………………………………………..33 LYING/STANDING AND FRUSEMIDE TEST………………………………………………..34 ADRENAL VENOUS SAMPLING……………………………………………………………..36 CHEMAI - Dynamic Function Protocol - 05 Page 2 of 47 Authorised by: -

MEC Fourth Edition Spread
Critères de recevabilité pour l'adoption et l'utilisation continue de méthodes contraceptives CHC Méthode de l’aménorrhée lactationnelle Stérilisation chirurgicale féminine Progestatifs seuls DIU Pilules pour la contraception d’urgence DIU Méthodes mécaniques Contraceptifs hormonaux combinés CHC Méthodes naturelles de planification familiale Coït interrompu StérilisationQuatrième chirurgicalegica édition, 2009 CHC Méthode de l’aménorrhée lactationnelle StérilisationGUIDE c ESSENTIELhirurgicale OMS DE PLANIFICATION FAMILIALE féminine Progestatifs seuls DIU Pilules pour la contraception d’urgence DIU Méthodes mécaniques Contraceptifs hormonaux combinés CHC Méthodes naturelles de planification familiale Coït interrompu Stérilisation chirurgicale CHC Méthode de l’aménorrhée lactationnelle Stérilisation chirurgicale féminine Progestatifs seuls DIU Pilules pour la contraception d’urgence DIU Méthodes mécaniques Contraceptifs hormonaux combinés CHC Méthodes naturelles de planification familiale Coït interrompu Stérilisation chirurgicale CHC Méthode de l’aménorrhée lactationnelle Stérilisation chirurgicale féminine Progestatifs seuls DIU Pilules pour la contraception d’urgence DIU Méthodes mécaniques Contraceptifs hormonaux combinés CHC Méthodes naturelles de planification familiale Coït interrompu Stérilisation chirurgicale Catalogage à la source: Bibliothèque de l’OMS: Critères de recevabilité pour l’adoption et l’utilisation continue de méthodes contraceptives -- 4e éd. 1.Contraception - méthodes. 2.Services de planification familiale. 3.Détermination -

Dalton Pharma Catalogue Steroids 12-19-12
2012/2013 Version: December 31, 2012 Steroid Analytical Reference Standards and Drug Impurities Dalton Pharma Services: A Health Canada Approved Facility Level-8 Controlled Substances License on-site SCC Approved GLP Facility North American Manufactured and Shipped To Order Call: 1.800.567.5060 To Order Call: 416.661.2102 Email: [email protected] Email: [email protected] Page 2 Dalton Standards and Impurities Table of Contents Introduction/Company Profile 4 Custom Synthesis Overview 6 Contract Research/Medicinal Chemistry 7 Process Development Overview 8 Analytical Services 9 GMP API & Sterile Filling Services 11 Formulation Development Overview 12 Liposomes 13 Dalton Standards and Impurities 14 Establishment License 16 Certificate example 17 Steroids 18 Page 3 To Order Call: 1.800.567.5060 To Order Email: [email protected] Call: Company Profile About Dalton Pharma Services Dalton Pharma Services is a privately-held pharmaceutical services company that has been producing Fine Chemi- cals on a custom basis for research and chemical supply houses for over twenty-five years (Fluka, Aldrich, Sigma, Acros). We have identified and supplied many new re- agents for biological and pharmaceutical applications, this includes novel analogues and impurities of active pharma- ceuticals ingredients, as well as new linkers. We are lead- ers in process development, process improvement, and in the field of isolation of kilo quantities of biologically active molecules from natural sources. Dalton excels at advancing projects from R&D into GMP environments for its customers. With the ability to manufacture cGMP API's from bench scales to multi kilos we can meet most clinical and small scale commercial requirements. -

For Selected Potent Endocrine Disrupting Steroids: Development and Application to Environmental Studies
Rapid and Sensitive Enzyme-Linked Immunosorbent Assays (ELISAs) for Selected Potent Endocrine Disrupting Steroids: Development and Application to Environmental Studies Chatchaporn Uraipong Thesis submitted in partial fulfillment of the requirement for the Degree of Master of Science (Research) School of Chemical Engineering The University of New South Wales September, 2010 ABSTRACT Endocrine disrupting chemicals (EDCs) are chemicals that alter functions of the endocrine system and cause health effects in an intact organism, or progeny, or population, with reproductive, developmental, or carcinogenic consequences. In order to facilitate risk assessment of potential endocrine disrupting steroids that are present in ultra low concentrations in the Australian environment, there is a need to boost the analytical capacity for EDC detection. One strategy is to develop antibody-based techniques that can offer simple, cost-effective and reliable analysis with high throughput capacity and portability for real-time monitoring. This thesis describes the design and synthesis of hapten molecules, raising of specific antibodies, formatting and characterising of a series of sensitive competitive Enzyme-Linked Immunosorbent Assays (ELISAs) for 17β-estradiol (E2), 17α-ethynylestradiol (EE2), ethylestradiol-3-methyl ether (mestranol) and testosterone (T), including validation of their performance as fast and effective water monitoring tools. Application of the developed assays to investigate the levels of the target EDCs in bodies of water and efficiency of water treatment plants in urban and rural areas in New South Wales, Australia, is also discussed. 17α-Ethynylestradiol and related synthetic estrogens, are active ingredients of contraceptive pills and hormone therapy, and have been identified as potent EDCs (Warner and Jenkins, 2007). -

Norethisterone and Ethinylestradiol
26 April 2019 EMA/245512/2019 - corr 1 Committee for Medicinal Products for Human Use (CHMP) Assessment report Procedure under Article 5(3) of Regulation (EC) No 726/2004 Norethisterone and ethinylestradiol Procedure number: EMEA/H/A-5(3)/1477 Note: Assessment report as adopted by the CHMP with all information of a commercially confidential nature deleted. Official address Domenico Scarlattilaan 6 ● 1083 HS Amsterdam ● The Netherlands Address for visits and deliveries Refer to www.ema.europa.eu/how-to-find-us Send us a question Go to www.ema.europa.eu/contact Telephone +31 (0)88 781 6000 An agency of the European Union © European Medicines Agency, 2019. Reproduction is authorised provided the source is acknowledged. Table of contents Table of contents ......................................................................................... 2 1. Information on the procedure ................................................................. 3 2. Scientific discussion ................................................................................ 3 2.1. Introduction ...................................................................................................... 3 2.2. Assessment of the meta-analysis .......................................................................... 4 2.3. Discussion of the meta-analysis ......................................................................... 25 3. Overall Conclusions ............................................................................... 28 4. References ........................................................................................... -

Master of $I)Ilof(Opl)P in 2Oolosp
BIOCHEMICAL AND CYTOGENETIC EFFECTS OF ORAL CONTRACEPTIVES AMONG WOMEN DISSERTATION SUBMITTED IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE AWARD OF THE DEGREE OF /^ Master of $I)ilof(opl)p in 2oolosp w By FALAQ NAZ o^ssf^^^-^''^io*--* ^ Under the Supervision of DR. YASIR HASAN SIDDIQUE SECTION OF GENETICS DEPARTMENT OF ZOOLOGY ALIGARH MUSLIM UNIVERSITY ALIGARH 2014 A iSyo sP:^ ^ <30 2 4 ;.CV 2014 DS4398 DROSOPHILA TRANSGENIC Dr. Yasir Hasan Siddique RESEARCH LABORATORY (Assistant Professor) E-mail: [email protected] CERTinCATE Certified that the thesis entitled ^Biochemical and Cytogenetic Effects of Oral Contraceptives among Women'' by Ms. Falaq naz for the partial fulfillment of the degree of Master of Philosophy (Zoology, Genetics) of the Aligarh Muslim University f Aligarh. The subject matter of dissertation as presented by Ms. Falaq Naz is the actual record of work done by her under my supen^sion and guidance, and the same does not form the part of any other course or degree presented to or taken by her. Dr./Yasir./Yasir Hasan SiSiddiquc e (Supervisor) Section of Genetics, Department of Zoology Aligarh Muslim University, Aligarh-202002, U.P., India DEDICATION TO THE LOVEUEST PARENTS IN THE WORLD WHO SUPPORT ME WITH AU. THEIR . HEARTS I? ' ^ TO TO MV DEAREST FRIENDS FOR THEIR h SUPPORT AND DUA IN HARD CIRCUMSTANCES TO MV UNIVERSITY WHICH IS WORKING TO IMPROVE THE RESEARCH TABLE OF COOTEFTS ACKNOWLEDGEMENTS 1-11 UST OF TABLES iii UST OF FIGURES iv ABBREVIATIONS v-vi 1. GENERAL INTRODUCTION 1-22 1.1. Female reproductive cycle 1 1.2. History of oral contraceptives 4 1.2.1. -

United States Patent (19) 11 Patent Number: 6,068,830 Diamandis Et Al
US00606883OA United States Patent (19) 11 Patent Number: 6,068,830 Diamandis et al. (45) Date of Patent: May 30, 2000 54) LOCALIZATION AND THERAPY OF FOREIGN PATENT DOCUMENTS NON-PROSTATIC ENDOCRINE CANCER 0217577 4/1987 European Pat. Off.. WITH AGENTS DIRECTED AGAINST 0453082 10/1991 European Pat. Off.. PROSTATE SPECIFIC ANTIGEN WO 92/O1936 2/1992 European Pat. Off.. WO 93/O1831 2/1993 European Pat. Off.. 75 Inventors: Eleftherios P. Diamandis, Toronto; Russell Redshaw, Nepean, both of OTHER PUBLICATIONS Canada Clinical BioChemistry vol. 27, No. 2, (Yu, He et al), pp. 73 Assignee: Nordion International Inc., Canada 75-79, dated Apr. 27, 1994. Database Biosis BioSciences Information Service, AN 21 Appl. No.: 08/569,206 94:393008 & Journal of Clinical Laboratory Analysis, vol. 8, No. 4, (Yu, He et al), pp. 251-253, dated 1994. 22 PCT Filed: Jul. 14, 1994 Bas. Appl. Histochem, Vol. 33, No. 1, (Papotti, M. et al), 86 PCT No.: PCT/CA94/00392 Pavia pp. 25–29 dated 1989. S371 Date: Apr. 11, 1996 Primary Examiner Yvonne Eyler S 102(e) Date: Apr. 11, 1996 Attorney, Agent, or Firm-Banner & Witcoff, Ltd. 87 PCT Pub. No.: WO95/02424 57 ABSTRACT It was discovered that prostate-specific antigen is produced PCT Pub. Date:Jan. 26, 1995 by non-proStatic endocrine cancers. It was further discov 30 Foreign Application Priority Data ered that non-prostatic endocrine cancers with Steroid recep tors can be stimulated with Steroids to cause them to produce Jul. 14, 1993 GB United Kingdom ................... 93.14623 PSA either initially or at increased levels.