Cellular Level Response of the Bivalve Limecola Balthica to Seawater
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Spatial Variability in Recruitment of an Infaunal Bivalve
Spatial Variability in Recruitment of an Infaunal Bivalve: Experimental Effects of Predator Exclusion on the Softshell Clam (Mya arenaria L.) along Three Tidal Estuaries in Southern Maine, USA Author(s): Brian F. Beal, Chad R. Coffin, Sara F. Randall, Clint A. Goodenow Jr., Kyle E. Pepperman, Bennett W. Ellis, Cody B. Jourdet and George C. Protopopescu Source: Journal of Shellfish Research, 37(1):1-27. Published By: National Shellfisheries Association https://doi.org/10.2983/035.037.0101 URL: http://www.bioone.org/doi/full/10.2983/035.037.0101 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Journal of Shellfish Research, Vol. 37, No. 1, 1–27, 2018. SPATIAL VARIABILITY IN RECRUITMENT OF AN INFAUNAL BIVALVE: EXPERIMENTAL EFFECTS OF PREDATOR EXCLUSION ON THE SOFTSHELL CLAM (MYA ARENARIA L.) ALONG THREE TIDAL ESTUARIES IN SOUTHERN MAINE, USA 1,2 3 2 3 BRIAN F. -

Cellular Level Response of the Bivalve Limecola Balthica To
Science of the Total Environment 794 (2021) 148593 Contents lists available at ScienceDirect Science of the Total Environment journal homepage: www.elsevier.com/locate/scitotenv Cellular level response of the bivalve Limecola balthica to seawater acidification due to potential CO2 leakage from a sub-seabed storage site in the southern Baltic Sea: TiTank experiment at representative hydrostatic pressure Adam Sokołowski a,1, Justyna Świeżak a,⁎,1, Anna Hallmann b, Anders J. Olsen c, Marcelina Ziółkowska a, Ida Beathe Øverjordet d, Trond Nordtug d, Dag Altin e, Daniel Franklin Krause d, Iurgi Salaberria c, Katarzyna Smolarz a a University of Gdańsk, Faculty of Oceanography and Geography, Institute of Oceanography, Al. Piłsudskiego 46, 81-378 Gdynia, Poland b Medical University of Gdańsk, Department of Pharmaceutical Biochemistry, Dębinki 1, 80-211 Gdańsk, Poland c Norwegian University of Science and Technology, NO-7491 Trondheim, Norway d SINTEF Ocean AS, Brattorkaia 17C, NO-7465 Trondheim, Norway e Altins Biotrix, Finn Bergs veg 3, 7022 Trondheim, Norway HIGHLIGHTS GRAPHICAL ABSTRACT • Cellular level responses of L. balthica to acidification caused by CO2 was tested at 9 ATM pressure. • The bivalve is tolerant to medium-term severe environmental hypercapnia. • Seawater pH 7.0 induced effects on rad- ical defence mechanisms (GPx, GST, CAT). • pH 6.3 caused increased cellular oxida- tive stress (MDA) and detoxification (tGSH). article info abstract Article history: Understanding of biological responses of marine fauna to seawater acidification due to potential CO2 leakage Received 7 April 2021 from sub-seabed storage sites has improved recently, providing support to CCS environmental risk assessment. Received in revised form 15 June 2021 Physiological responses of benthic organisms to ambient hypercapnia have been previously investigated but Accepted 17 June 2021 rarely at the cellular level, particularly in areas of less common geochemical and ecological conditions such as Available online 24 June 2021 brackish water and/or reduced oxygen levels. -

HETA ROUSI: Zoobenthos As Indicators of Marine Habitats in the Northern Baltic
Heta Rousi Zoobenthos as indicators of marine Heta Rousi | habitats in the northern Baltic Sea of marine as indicators habitats in the northernZoobenthos Baltic Sea Heta Rousi This thesis describes how physical and chemical environmental variables impact zoobenthic species distribution in the northern Baltic Sea and how dis- Zoobenthos as indicators of marine tinct zoobenthic species indicate different marine benthic habitats. The thesis inspects the effects of habitats in the northern Baltic Sea depth, sediment type, temperature, salinity, oxy- gen, nutrients as well as topographical and geo- logical factors on zoobenthos on small and large temporal and spatial scales. | 2020 ISBN 978-952-12-3944-1 Heta Rousi Född 1979 Studier och examina Magister vid Helsingfors Universitet 2006 Licentiat vid Åbo Akademi 2013 Doktorsexamen vid Åbo Akademi 2020 Institutionen för miljö- och marinbiologi, Åbo Akademi ZOOBENTHOS AS INDICATORS OF MARINE HABITATS IN THE NORTHERN BALTIC SEA HETA ROUSI Environmental and Marine Biology Faculty of Science and Engineering Åbo Akademi University Finland, 2020 SUPERVISED BY PRE-EXAMINED BY Professor Erik Bonsdorff Research Professor (Supervisor & Examiner) Markku Viitasalo Åbo Akademi University Finnish Environment Institute Faculty of Science and Engineering Sustainable Use of the Marine Areas Environmental and Marine Biology Latokartanonkaari 11 Artillerigatan 6 00790 Helsinki 20520 Åbo Finland Finland Professor Emeritus Ilppo Vuorinen CO-SUPERVISOR University of Turku Adjunct Professor Faculty of Science and Engineering Samuli Korpinen Itäinen Pitkäkatu 4 Finnish Environment Institute 20520 Turku Marine Management Finland Latokartanonkaari 11 00790 Helsinki FACULTY OPPONENT Finland Associate Professor Urszula Janas SUPERVISING AT THE University of Gdansk LICENCIATE PHASE Institute of Oceanography Assistant Professor Al. -

Elemental Composition of Invertebrates Shells Composed Of
https://doi.org/10.5194/bg-2019-367 Preprint. Discussion started: 20 September 2019 c Author(s) 2019. CC BY 4.0 License. 1 Elemental composition of invertebrates shells composed of different CaCO3 polymorphs at 2 different ontogenetic stages: a case study from the brackish Gulf of Gdansk (the Baltic Sea) 3 4 Anna Piwoni-Piórewicz1*, Stanislav Strekopytov2†, Emma Humphreys-Williams2, Piotr 5 Kukliński1, 3 6 7 1Institute of Oceanology, Polish Academy of Sciences, Powstańców Warszawy 55, 81-712 8 Sopot, Poland 9 2Imaging and Analysis Centre, Natural History Museum, Cromwell Road, London SW7 5BD, 10 United Kingdom 11 3Department of Life Sciences, Natural History Museum, Cromwell Road, London SW7 5BD, 12 United Kingdom 13 14 *Corresponding author 15 E-mail: [email protected] 16 Tel.: (+ 48 58) 731 16 96 17 † Present address: Inorganic Analysis, LGC Ltd, Queens Road, Teddington, United Kingdom 18 19 Abstract 20 In this study, the concentrations of 12 metals: Ca, Na, Sr, Mg, Ba, Mn, Cu, Pb, V, Y, U and 21 Cd in shells of bivalve molluscs (aragonitic: Cerastoderma glaucum, Mya arenaria and 22 Limecola balthica and bimineralic: Mytilus trossulus) and arthropods (calcitic: Amphibalanus 23 improvisus) were obtained. The main goal was to determine the incorporation patterns of 24 shells built with different calcium carbonate polymorphs. The role of potential biological 25 control on the shell chemistry was assessed by comparing the concentrations of trace elements 26 between younger and older individuals (different size classes). The potential impact of 27 environmental factors on the observed elemental concentrations in the studied shells is 28 discussed. -

Download PDF Version
MarLIN Marine Information Network Information on the species and habitats around the coasts and sea of the British Isles Baltic tellin (Limecola balthica) MarLIN – Marine Life Information Network Biology and Sensitivity Key Information Review Georgina Budd & Will Rayment 2001-12-19 A report from: The Marine Life Information Network, Marine Biological Association of the United Kingdom. Please note. This MarESA report is a dated version of the online review. Please refer to the website for the most up-to-date version [https://www.marlin.ac.uk/species/detail/1465]. All terms and the MarESA methodology are outlined on the website (https://www.marlin.ac.uk) This review can be cited as: Budd, G.C.& Rayment, W.J. 2001. Limecola balthica Baltic tellin. In Tyler-Walters H. and Hiscock K. (eds) Marine Life Information Network: Biology and Sensitivity Key Information Reviews, [on-line]. Plymouth: Marine Biological Association of the United Kingdom. DOI https://dx.doi.org/10.17031/marlinsp.1465.1 The information (TEXT ONLY) provided by the Marine Life Information Network (MarLIN) is licensed under a Creative Commons Attribution-Non-Commercial-Share Alike 2.0 UK: England & Wales License. Note that images and other media featured on this page are each governed by their own terms and conditions and they may or may not be available for reuse. Permissions beyond the scope of this license are available here. Based on a work at www.marlin.ac.uk (page left blank) Date: 2001-12-19 Baltic tellin (Limecola balthica) - Marine Life Information Network See online review for distribution map Limecola balthica. -

Tisja Daggers ISBN: 978-90-365-5124-3 Tisja Daggers DOI: 10.3990/1.9789036551243 Tisja Daggers Diss
Quantifying intertidal sensing optical using in temperate the ecosystems remote role of microphytobenthos INVITATION Quantifying the role of microphytobenthos in temperate intertidal ecosystems using I have the pleasure of inviting you to attend optical remote sensing the public defence of my dissertation entitled: Quantifying the role of microphytobenthos in temperate intertidal ecosystems using optical remote sensing Which will take place on Thursday 25th of February De Waaier, room 4, University of Twente, Enschede 14.30 Layman’s talk 14.45 PhD defence Tisja DaggersTisja ISBN: 978-90-365-5124-3 Tisja Daggers DOI: 10.3990/1.9789036551243 Tisja Daggers Diss. no. 391 QUANTIFYING THE ROLE OF MICROPHYTOBENTHOS IN TEMPERATE INTERTIDAL ECOSYSTEMS USING OPTICAL REMOTE SENSING Tisja Daggers This dissertation has been approved by: Supervisors prof. dr. D. van der Wal prof. dr. P.M.J. Herman Cover design: Job Duim and Tisja Daggers (painting) Printed by: ITC Printing Department Lay-out: Tisja Daggers ISBN: 978-90-365-5124-3 DOI: 10.3990/1.9789036551243 This research was carried out at NIOZ Royal Netherlands Institute for Sea Research and financially supported by the ‘User Support Programme Space Research’ of the Netherlands Organisation for Scientific Research. © 2021 Tisja Dorine Daggers/ NIOZ, The Netherlands. All rights reserved. No parts of this thesis may be reproduced, stored in a retrieval system or transmitted in any form or by any means without permission of the author. Alle rechten voorbehouden. Niets uit deze uitgave mag worden vermenigvuldigd, in enige vorm of op enige wijze, zonder voorafgaande schriftelijke toestemming van de auteur. QUANTIFYING THE ROLE OF MICROPHYTOBENTHOS IN TEMPERATE INTERTIDAL ECOSYSTEMS USING OPTICAL REMOTE SENSING DISSERTATION to obtain the degree of doctor at the Universiteit Twente, on the authority of the rector magnificus, prof. -
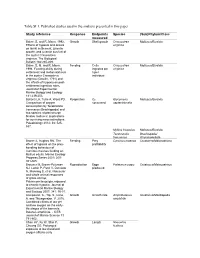
Table SI 1. Published Studies Used in the Analysis Presented in This Paper
Table SI 1. Published studies used in the analysis presented in this paper. Study reference Response Endpoints Species (Sub)Phylum/Class measured Baker, S. and R. Mann. 1992. Growth Shell growth Crassostrea Mollusca/Bivalvia Effects of hypoxia and anoxia virginica on larval settlement, juvenile growth, and juvenile survival of the oyster Crassostrea virginica. The Biological Bulletin 182:265-269. Baker, S. M. and R. Mann. Feeding Cells Crassostrea Mollusca/Bivalvia 1994. Feeding ability during ingested per virginica settlement and metamorphosis h per in the oyster Crassostrea individual virginica (Gmelin, 1791) and the effects of hypoxia on post- settlement ingestion rates. Journal of Experimental Marine Biology and Ecology 181:239-253. Ballanti LA, Tullis A, Ward PD. Respiration O2 Glycymeris Mollusca/Bivalvia Comparison of oxygen consumed septentrionalis consumption by Terebratalia transversa (Brachiopoda) and two species of pteriomorph bivalve molluscs: implications for surviving mass extinctions. Paleobiology 2012; 38: 525- 537. Mytilus trossulus Mollusca/Bivalvia Terebratalia Brachiopoda/ transversa Rhynchonellata Brante A, Hughes RN. The Feeding Prey Carcinus maenas Crustacea/Malacostraca effect of hypoxia on the prey- profitability handling behaviour of Carcinus maenas feeding on Mytilus edulis. Marine Ecology Progress Series 2001; 209: 301-305. Brouwer M, Brown-Peterson Reproduction Eggs Palaemon pugio Crustacea/Malacostraca NJ, Larkin P, Patel V, Denslow produced N, Manning S, et al. Molecular and whole animal responses of grass shrimp, Palaemonetes pugio, exposed to chronic hypoxia. Journal of Experimental Marine Biology and Ecology 2007; 341: 16-31. Campanati, C., Yip, S., Lane, Growth Growth rate Amphibalanus Crustacea/Maxillopoda A. and Thiyagarajan, V. 2016. amphitrite Combined effects of low pH and low oxygen on the early- life stages of the barnacle Balanus amphitrite. -

Investigation of the Molecular Signatures of Selection on ATP
Investigation of the molecular signatures of selection on ATP synthase genes in the marine bivalve Limecola balthica Eric Pante, Vanessa Becquet, Amélia Viricel, Pascale Garcia To cite this version: Eric Pante, Vanessa Becquet, Amélia Viricel, Pascale Garcia. Investigation of the molecular signatures of selection on ATP synthase genes in the marine bivalve Limecola balthica. Aquatic Living Resources, EDP Sciences, 2019, 32, pp.3. 10.1051/alr/2019001. hal-02383588 HAL Id: hal-02383588 https://hal-univ-rochelle.archives-ouvertes.fr/hal-02383588 Submitted on 8 Nov 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. 1 Investigation of the molecular signatures of selection on ATP synthase genes in the marine 2 bivalve Limecola balthica 3 Eric Pante1*, Vanessa Becquet1, Amélia Viricel1, and Pascale Garcia1 4 1 Littoral, Environnement et Sociétés (LIENSs), UMR 7266 CNRS – Université de La Rochelle, 2 rue 5 Olympe de Gouges, 17000 La Rochelle, France ∗ Corresponding author. E-mail: [email protected]; 6 Phone: +33.05.46.50.76.37; Fax: +33.05.46.50.76.63. 7 Abstract 8 We used transcriptomic sequence data to describe patterns of divergence and selection across 9 different populations of a marine bivalve (Limecola balthica). -

Biochemical Characterisation of Potentially Contagious Haemic Neoplasia Occurring in the Baltic Clam Limecola Balthica
Biochemical characterisation of potentially contagious haemic neoplasia occurring in the Baltic clam Limecola balthica Hallmann Anna Medical University of Gdańsk Michnowska Alicja University of Gdańsk Chomiczewska Agnieszka Medical University of Gdańsk Lipiński Marcin Medical University of Gdańsk Smolarz Katarzyna ( [email protected] ) University of Gdańsk Research Article Keywords: leukemia-like neoplasia, energy homeostasis, FAA, ROS, mitochondrial respiration, Limecola balthica Posted Date: April 29th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-468774/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Biochemical characterisation of potentially contagious haemic neoplasia occurring in the Baltic clam Limecola balthica Hallmann Anna1, Michnowska Alicja2, Chomiczewska Agnieszka1,3, Lipiński Marcin1, Smolarz Katarzyna2* 1. Department of Pharmaceutical Biochemistry, Medical University of Gdańsk, Gdańsk, Poland 2. Department of Oceanography and Geography, University of Gdańsk, Gdynia, Poland 3. Department of Biochemistry, Medical University of Gdańsk, Gdańsk, Poland Contact person: *Smolarz Katarzyna, email address [email protected] Key words: leukemia-like neoplasia, energy homeostasis, FAA, ROS, mitochondrial respiration, Limecola balthica 1 Abstract Occurring in marine invertebrates haemic (leukemia-like) neoplasia, a disease of potentially infectious nature, arises from genome instabilities leading to multilevel malfunctions and unregulated cell division of presumably haemocyte precursors. As its biochemical characterisation remains unknown, we here present results describing selected physiological and biochemical aspects of the disease measured in neoplastic and healthy clams Limecola balthica. Activities of adenosine deaminase (ADA), alkaline phosphatase (ALP) and FAA levels were measured in haemolymph but no differences in ADA and ALP activities between healthy and neoplastic clams were found. -

Ruditapes Hybrids
EIDO Escola Internacional de Doutoramento TESE DE DOUTORAMENTO Caracterización citogenética de bivalvos veneroideos y algunos de sus parásitos Cytogenetic characterization of veneroid bivalves and some of their parasites Daniel García Souto 2017 Mención internacional Aquéllos que me busquen, sabrán que he existido. Los demás no tienen necesidad de saberlo. Gustav Mahler AGRADECIMIENTOS Estimado lector anónimo, sea Ud. consciente de que, más allá de la loable función de calzar algún que otro mueble cojo, esta tesis es la consecución, el pináculo quizás, de una etapa de considerable esfuerzo en mi vida. Pero ante todo, sepa que se encuentra ante el trabajo de muchas personas. Esta es, en gran parte la (enésima) tesis de Juanjo, mi director y de Paloma, mi social manager. Ambos han tenido a bien aguantarme un buen rato y, siendo francos, han contribuido tanto (o más) que uno mismo al presente manuscrito. Creo, sinceramente que me habéis ayudado a madurar como investigador y como persona. Espero haber estado a la altura de vuestras expectativas. A ambos muchas gracias. Tras las presentes páginas subyace además el trabajo colaborativo de todo un departamento cuyos integrantes, incluso de forma inconsciente, han contribuido a hacer de mi estancia en el bucólico CUVIlete más cómoda, más agradable y, en definitiva, mejor. Gracias pues a todos los que están y a los que en su momento estuvieron. Esta es también la tesis de los Erasmus-party de la Universidad de Portsmouth (en estricto orden de aparición Neil, Jack, Vesa y Auriel) y de los ocupas de turno del grado en Biología (Jonathan, Pablo, Sonia, Yaiza, Sandra, Susana, Gonzalo y Agustín). -

BAB IV HASIL PENELITIAN DAN PEMBAHASAN A. Hasil Dan Pembahasan Penelitian (Tahap I) 1. Gambaran Lokasi Penelitian Pantai Pasir P
BAB IV HASIL PENELITIAN DAN PEMBAHASAN A. Hasil dan Pembahasan Penelitian (Tahap I) 1. Gambaran Lokasi Penelitian Pantai Pasir Putih merupakan salah satu dari berbagai obyek wisata yang berlokasi di Kabupaten Trenggalek. Pantai tersebut tersusun dari daratan berupa pasir putih dan lautan berbentuk cekungan yang daerah pesisirnya terdapat dinding karang dari ujung barat sampai ujung timur. Pantai Pasir Putih terletak di Desa Tasikmadu Kecamatan Watulimo sekitar 48 km dari pusat kota Trenggalek dengan panjang pantai 1,5 km. Adapun peta lokasi Kabupaten Trenggalek dapat dilihat pada Gambar 4.1 sebagai berikut. Gambar 4.1 Peta Kabupaten Trenggalek 73 74 Pantai Karanggongso merupakan nama lain dari Pantai Pasir Putih dan sangat terkenal dikalangan masyarakat pada umumnya. Pantai Karanggongso adalah pantai dengan pasir berwarna putih yang bersih dan warna laut yang membiru indah. Adapun peta lokasi Pantai Pasir Putih yang dapat dilihat pada Gambar 4.2 sebagai berikut. Gambar 4.2 Peta Pantai Pasir Putih1 2. Faktor Abiotik Bivalvia memiliki tingkat toleransi terhadap kondisi lingkungan seperti suhu, salinitas, dan derajat keasaman (pH) yang bervariasi selama periode hidupnya.2 Hasil pengukuran faktor abiotik yang dilakukan pada 15 plot penelitian memiliki nilai rata-rata pada Tabel 4.1 sebagai berikut. 1https://www.google.co.id/maps/place/Pantai+Pasir+Putih+Karanggongso/ (diakses pada 10 Juni 2020, pukul 22:08) 2Muhammad Masrur Islami, Pengaruh Suhu Dan Salinitas Terhadap Bivalvia, (Oseana, Volume XXXVIII, Nomor 2, 2013), hal. 2 75 Tabel 4.1 -
Putative Sex-Linked Heteroplasmy in the Tellinid Bivalve Limecola Balthica
Putative sex-linked heteroplasmy in the tellinid bivalve Limecola balthica (Linnaeus, 1758) Eric Pante, Camille Poitrimol, Alice Saunier, Vanessa Becquet, Pascale Garcia To cite this version: Eric Pante, Camille Poitrimol, Alice Saunier, Vanessa Becquet, Pascale Garcia. Putative sex-linked heteroplasmy in the tellinid bivalve Limecola balthica (Linnaeus, 1758). Journal of Molluscan Studies, Oxford University Press (OUP), 2017, 83 (2), pp. 226-228. 10.1093/mollus/eyw038. hal-01390664 HAL Id: hal-01390664 https://hal.archives-ouvertes.fr/hal-01390664 Submitted on 4 Nov 2016 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. 1 RESEARCH NOTE 2 3 Putative sex-linked heteroplasmy in the tellinid bivalve Limecola balthica (Linnaeus, 1758) 4 5 Eric Pante1, Camille Poitrimol, Alice Saunier, Vanessa Becquet and Pascale Garcia 6 LIENSs Laboratory, UMR7266 CNRS – University of La Rochelle, 7 2 Rue Olympe de Gouges, 17000 La Rochelle, France 8 Correspondence: E. Pante; email: [email protected] 9 10 Doubly uniparental inheritance (DUI) is a remarkable exception to the maternal inheritance of 11 mitochondria in metazoans. Found in bivalves, DUI is characterized by females transmitting ‘female’ 12 (F) mitochondria that persist in oocytes and in somatic tissues of both sexes, whereas males pass on 13 ‘male’ (M) mitochondria that persist in the male germ line (reviewed by Zouros, 2013).