Maize Dwarf Mosaic Virus CP
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Determining the Role of Seed and Soil in the Transmission Of
DETERMINING THE ROLE OF SEED AND SOIL IN THE TRANSMISSION OF VIRUSES CAUSING MAIZE LETHAL NECROSIS DISEASE By GATUNZI KITIRA FELIX (B.Sc. in Rural Development) A thesis submitted in partial fulfillment of requirements for the award of degree of Master of Science in Plant Pathology Department of Plant Science and Crop Protection Faculty of Agriculture University of Nairobi 2018 DECLARATION This thesis is a presentation of my original research work and has not been presented for a degree in any other University. Felix Gatunzi Kitira Date…................................................ This thesis has been submitted with our approval as University supervisors: Dr. Douglas Watuku Miano Department of Plant Science and Crop Protection University of Nairobi Signature ….…………………………. Date……………………………….. Prof. Daniel Mukunya Department of Plant Science and Crop Protection Signature ……………………………. Date……………………………….. Dr. Suresh Lingadahalli Mahabaleswara Global Maize Program (GMP), International Maize and Wheat Improvement Center CIMMYT Signature.…………………………………… Date……………………………… i PLAGIARISM DECLARATION I understand what plagiarism is and I am aware of the University’s policy. In this regard, I declare that this MSc Thesis is my original work. Where other people’s work or my own work has been used, it has properly been acknowledged and referenced in accordance with the University of Nairobi’s requirement. I have not allowed, and shall allow anyone to copy my work with the intention of passing it off as his or her own work. I understand that any false claim in respect of this work shall result in disciplinary action, in accordance with University Plagiarism Policy. Signature.................................................................Date.......................................................... ii DEDICATION To the almighty God for imparting me with his grace along this journey. -

Aphid Transmission of Potyvirus: the Largest Plant-Infecting RNA Virus Genus
Supplementary Aphid Transmission of Potyvirus: The Largest Plant-Infecting RNA Virus Genus Kiran R. Gadhave 1,2,*,†, Saurabh Gautam 3,†, David A. Rasmussen 2 and Rajagopalbabu Srinivasan 3 1 Department of Plant Pathology and Microbiology, University of California, Riverside, CA 92521, USA 2 Department of Entomology and Plant Pathology, North Carolina State University, Raleigh, NC 27606, USA; [email protected] 3 Department of Entomology, University of Georgia, 1109 Experiment Street, Griffin, GA 30223, USA; [email protected] * Correspondence: [email protected]. † Authors contributed equally. Received: 13 May 2020; Accepted: 15 July 2020; Published: date Abstract: Potyviruses are the largest group of plant infecting RNA viruses that cause significant losses in a wide range of crops across the globe. The majority of viruses in the genus Potyvirus are transmitted by aphids in a non-persistent, non-circulative manner and have been extensively studied vis-à-vis their structure, taxonomy, evolution, diagnosis, transmission and molecular interactions with hosts. This comprehensive review exclusively discusses potyviruses and their transmission by aphid vectors, specifically in the light of several virus, aphid and plant factors, and how their interplay influences potyviral binding in aphids, aphid behavior and fitness, host plant biochemistry, virus epidemics, and transmission bottlenecks. We present the heatmap of the global distribution of potyvirus species, variation in the potyviral coat protein gene, and top aphid vectors of potyviruses. Lastly, we examine how the fundamental understanding of these multi-partite interactions through multi-omics approaches is already contributing to, and can have future implications for, devising effective and sustainable management strategies against aphid- transmitted potyviruses to global agriculture. -

A New Rhabdo Virus Infection in Maize Plantations in Turkey
Global Advanced Research Journal of Agricultural Science (ISSN: 2315-5094) Vol. 6(9) pp. 310-313, September, 2017 Issue. Available online http://garj.org/garjas/home Copyright © 2017 Global Advanced Research Journals Full Length Research Paper A new Rhabdo virus Infection in Maize Plantations in Turkey Filiz Ertunc Ankara University, Faculty of Agriculture, Department of Plant Protection, 06110 Ankara,Turkey Email:[email protected] Accepted 05 October, 2017 Maize mosaicrhabdo virus (MMV) is devastating virus infection, causes serious crop losses, on the family Poaceae is especially on maize and transmitedby leaf hopper, Peregrinusmaidis. Samples were collected from leaves and stems of maize plants, from Bartın, Düzce, Sakarya and Zonguldak provinces of Turkey, in 2012-2013. They were tested against MMV antiserum by DAS-ELISA and also RT-PCR assay with a primers specific to glycoprotein gene of the virus, designed in our laboratory. 15 of the collected maize samples were found to be infected with MMV. Positive samples were mechanically inoculated to maize plant lets for propagation and maintain. MMV inoculated maize plants, show light mosaic symptoms were tested by ELISA and RT-PCR again, obtained bands were 470bp long. In electron microscopic observations, rhabdovirus-like particules were detected.So far to our knowledge, it is the first report of Maize mosaic rhabdovirus presence in Turkey. Keywords: Maize, MMV, Rhabdovirus, RT-PCR, electromicrocopy INTRODUCTION Maize ( Zea mays L.) is a culture plant, belonging to on the cobs of the infected maize plants. More than forty Poaceae family, and is grown as food and feed in Turkey. virus infections are detected in corn plantations in the Turkey is one of the main producter of maize and it is Mediterranean basin and in the world (Ivanovic et al. -

Maize Dwarf Mosaic Virus: from Genome to Disease Management
viruses Review Maize Dwarf Mosaic Virus: From Genome to Disease Management Maathavi Kannan 1, Ismanizan Ismail 1,2 and Hamidun Bunawan 1,* 1 Institute of Systems Biology, Universiti Kebangsaan Malaysia, 43600 Bangi, Malaysia; [email protected] (M.K.); [email protected] (I.I.) 2 School of Bioscience and Biotechnology, Faculty of Science and Technology, Universiti Kebangsaan Malaysia, 43600 Bangi, Malaysia * Correspondence: [email protected]; Tel.: +60-3-8921-4554 Received: 24 July 2018; Accepted: 28 August 2018; Published: 13 September 2018 Abstract: Maize dwarf mosaic virus (MDMV) is a serious maize pathogen, epidemic worldwide, and one of the most common virus diseases for monocotyledonous plants, causing up to 70% loss in corn yield globally since 1960. MDMV belongs to the genus Potyvirus (Potyviridae) and was first identified in 1964 in Illinois in corn and Johnsongrass. MDMV is a single stranded positive sense RNA virus and is transmitted in a non-persistent manner by several aphid species. MDMV is amongst the most important virus diseases in maize worldwide. This review will discuss its genome, transmission, symptomatology, diagnosis and management. Particular emphasis will be given to the current state of knowledge on the diagnosis and control of MDMV, due to its importance in reducing the impact of maize dwarf mosaic disease, to produce an enhanced quality and quantity of maize. Keywords: Maize dwarf mosaic virus; genome; transmission; symptomatology; diagnosis; management 1. Introduction Maize (Zea mays L.) is one of the most highly cultivated crops worldwide. It is the third largest crop grown in the developing world and it has been identified as a major staple food in Africa [1]. -

Sugarcane Mosaic Virus Infects Stenotaphrum Secundatum in Australia
Australasian Plant Disease Notes (2020) 15:41 https://doi.org/10.1007/s13314-020-00410-y Sugarcane mosaic virus infects Stenotaphrum secundatum in Australia Nga T. Tran1 & Ai Chin Teo1 & John E. Thomas1 & Kathleen S. Crew1,2 & Andrew D. W. Geering1 Received: 28 September 2020 /Accepted: 2 November 2020 # Australasian Plant Pathology Society Inc. 2020 Abstract This study presents the first report of sugarcane mosaic virus (SCMV) infecting Stenotaphrum secundatum (buffalo grass) in Australia, from a turf farm in the Hunter Valley, New South Wales. The plant displayed mosaic symptoms and contained flexuous, filamentous virions of 700–750 × 10–11 nm typical of members of the genus Potyvirus. Infection of the sample by SCMV was confirmed by double antibody sandwich ELISA and RT-PCR amplification of the coat protein coding region of the viral genome. In a phylogenetic analysis, the buffalo grass isolate was sister to a clade of maize-infecting isolates of SCMV from eastern Africa and was 75.8% and 79.4% identical to the exemplar isolate of SCMV at nucleotide and amino acid levels, respectively. Keywords Potyvirus . Buffalo grass . St. Augustinegrass . Nucleotide sequence Stenotaphrum secundatum (buffalo grass in Australia or St. SCMV has a worldwide distribution and infects several Augustinegrass in the USA) is a hardwearing and vigorous economically important monocotyledonous crops including warm season turfgrass species, thought to be endemic to the maize (Zea mays), sorghum (Sorghum bicolor) and sugarcane Atlantic coasts of the Americas and Africa (Sauer 1972; (Saccharum officinarum) (Yang and Mirkov 1997;Wuetal. Busey 2003). It is the most important turf species in 2012). -

Edna-Host: Detection of Global Plant Viromes Using High Throughput Sequencing
EDNA-HOST: DETECTION OF GLOBAL PLANT VIROMES USING HIGH THROUGHPUT SEQUENCING By LIZBETH DANIELA PENA-ZUNIGA Bachelor of Science in Biotechnology Escuela Politecnica de las Fuerzas Armadas (ESPE) Sangolqui, Ecuador 2014 Submitted to the Faculty of the Graduate College of the Oklahoma State University in partial fulfillment of the requirements for the Degree of DOCTOR OF PHILOSOPHY May 2020 EDNA-HOST: DETECTION OF GLOBAL PLANT VIROMES USING HIGH THROUGHPUT SEQUENCING Dissertation Approved: Francisco Ochoa-Corona, Ph.D. Dissertation Adviser Committee member Akhtar, Ali, Ph.D. Committee member Hassan Melouk, Ph.D. Committee member Andres Espindola, Ph.D. Outside Committee Member Daren Hagen, Ph.D. ii ACKNOWLEDGEMENTS I would like to express sincere thanks to my major adviser Dr. Francisco Ochoa –Corona for his guidance from the beginning of my journey believing and trust that I am capable of developing a career as a scientist. I am thankful for his support and encouragement during hard times in research as well as in personal life. I truly appreciate the helpfulness of my advisory committee for their constructive input and guidance, thanks to: Dr. Akhtar Ali for his support in this research project and his kindness all the time, Dr. Hassan Melouk for his assistance, encouragement and his helpfulness in this study, Dr. Andres Espindola, developer of EDNA MiFi™, he was extremely helpful in every step of EDNA research, and for his willingness to give his time and advise; to Dr. Darren Hagen for his support and advise with bioinformatics and for his encouragement to develop a new set of research skills. I deeply appreciate Dr. -

Effects of Plant Viruses on Vectors and Non-Vector Herbivores in Three
Louisiana State University LSU Digital Commons LSU Doctoral Dissertations Graduate School March 2019 Effects of Plant Viruses on Vectors and Non-vector Herbivores in Three Different Pathosystems Sunil Paudel Louisiana State University and Agricultural and Mechanical College, [email protected] Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_dissertations Part of the Entomology Commons Recommended Citation Paudel, Sunil, "Effects of Plant Viruses on Vectors and Non-vector Herbivores in Three Different Pathosystems" (2019). LSU Doctoral Dissertations. 4870. https://digitalcommons.lsu.edu/gradschool_dissertations/4870 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Doctoral Dissertations by an authorized graduate school editor of LSU Digital Commons. For more information, please [email protected]. EFFECTS OF PLANT VIRUSES ON VECTORS AND NON-VECTOR HERBIVORES IN THREE DIFFERENT PATHOSYSTEMS A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Entomology by Sunil Paudel B. S., Tribhuvan University, 2008 M.S., University of Idaho, 2013 May 2019 ACKNOWLEDGEMENTS I would like to express my sincere gratitude to my major advisor, Dr. Jeffrey A. Davis for his continuous support throughout my graduate studies, for his patience, encouragement, and guidance that helped me to grow as a researcher and a critical thinker. I am also thankful to my dissertation committee members, Drs. Michael J. Stout, Fangneng Huang, Jeffrey W. Hoy, and Dean’s representative Dr. -
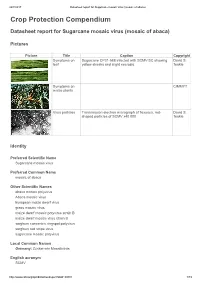
Datasheet Report for Sugarcane Mosaic Virus (Mosaic of Abaca)
24/7/2017 Datasheet report for Sugarcane mosaic virus (mosaic of abaca) Crop Protection Compendium Datasheet report for Sugarcane mosaic virus (mosaic of abaca) Pictures Picture Title Caption Copyright Symptoms on Sugarcane CP31-588 infected with SCMV-SC showing David S. leaf yellow streaks and slight necrosis. Teakle Symptoms on CIMMYT maize plants Virus particles Transmission electron micrograph of flexuous, rod- David S. shaped particles of SCMV x40 000. Teakle Identity Preferred Scientific Name Sugarcane mosaic virus Preferred Common Name mosaic of abaca Other Scientific Names abaca mosaic potyvirus Abaca mosaic virus European maize dwarf virus grass mosaic virus maize dwarf mosaic potyvirus strain B maize dwarf mosaic virus strain B sorghum concentric ringspot potyvirus sorghum red stripe virus sugarcane mosaic potyvirus Local Common Names Germany: Zuckerrohr Mosaikvirus English acronym SCMV http://www.cabi.org/cpc/datasheetreport?dsid=49801 1/19 24/7/2017 Datasheet report for Sugarcane mosaic virus (mosaic of abaca) EPPO code SCMV00 (Sugarcane mosaic potyvirus) Taxonomic Tree Domain: Virus Group: "Positive sense ssRNA viruses" Group: "RNA viruses" Family: Potyviridae Genus: Potyvirus Species: Sugarcane mosaic virus Notes on Taxonomy and Nomenclature This virus was described from sugarcane by Brandes (1919). Strains naturally infecting certain other hosts are also known as abaca mosaic potyvirus (Eloja and Tinsley, 1963) and as maize dwarf mosaic potyvirus strain B (Mackenzie et al., 1966; Louie and Knoke, 1975). Until recently, most aphid-borne potyviruses infecting sugarcane and other members of the Poaceae were assumed to be either sugarcane mosaic virus or maize dwarf mosaic virus. However, new criteria for speciation of these members of the Potyviridae have been proposed (Shukla et al., 1989; Shukla et al., 1992; Shukla and Ward, 1994): the amino-acid sequence homology of their coat proteins, and serological relationships with antisera derived from epitopes in the amino terminus rather than the core region of the coat protein. -

Occurrence and Importance of Maize Dwarf Mosaic Virus in Massachusetts
University of Massachusetts Amherst ScholarWorks@UMass Amherst Masters Theses 1911 - February 2014 1973 Occurrence and importance of maize dwarf mosaic virus in Massachusetts. Robert A. Schall University of Massachusetts Amherst Follow this and additional works at: https://scholarworks.umass.edu/theses Schall, Robert A., "Occurrence and importance of maize dwarf mosaic virus in Massachusetts." (1973). Masters Theses 1911 - February 2014. 3285. Retrieved from https://scholarworks.umass.edu/theses/3285 This thesis is brought to you for free and open access by ScholarWorks@UMass Amherst. It has been accepted for inclusion in Masters Theses 1911 - February 2014 by an authorized administrator of ScholarWorks@UMass Amherst. For more information, please contact [email protected]. OCCURRENCE AND IMPORTANCE OF MAIZE DWARF MOSAIC VIRUS IN MASSACHUSETTS ROBERT A. SCHALL B. A. Rutgers University Newark, New Jersey Thesis submitted to the Graduate Faculty in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE University of Massachusetts Department of Plant Pathology Amherst, Massachusetts July, 1973 OCCURRENCE AND IMPORTANCE OF MAIZE DWARF MOSAIC VIRUS IN MASSACHUSETTS A Thesis by Robert A. Schall Approved as to style and content by: (Member) (Month) ; (Year) ACKNOWLEDGEMENTS I am deeply grateful to my advisor Dr. George N. Agrios for his constant encouragement and advice throughout this work and for his assistance in the preparation of this manuscript. I am also indebted to Professors Cecil L. Thomson and William H. Lachman for assisting me in this work with their comprehensive knowledge of corn culture and production. Also, I wish to thank Messrs. Dominic Marini, Walter Melnick, and Alden Miller who, in their capacity as Exten¬ sion Specialists, helped me survey for the virus. -

Control of Plant Viral Diseases by CRISPR/Cas9: Resistance Mechanisms, Strategies and Challenges in Food Crops
plants Review Control of Plant Viral Diseases by CRISPR/Cas9: Resistance Mechanisms, Strategies and Challenges in Food Crops Saleh Ahmed Shahriar 1, M. Nazrul Islam 2 , Charles Ng Wai Chun 3, Md. Abdur Rahim 4 , Narayan Chandra Paul 5 , Jasim Uddain 6 and Shafiquzzaman Siddiquee 7,* 1 School of Biological Sciences, Universiti Sains Malaysia, Penang 11800, Malaysia; [email protected] 2 Laboratory of Plant Pathology and Microbiology, Morden Research and Development Centre, Agriculture and Agri-Food Canada, Morden, MB R6M 1Y5, Canada; [email protected] 3 Bioprocess Technology Division, School of Industrial Technology, Universiti Sains Malaysia, Penang 11800, Malaysia; [email protected] 4 Department of Genetics and Plant Breeding, Sher-e-Bangla Agricultural University, Dhaka 1207, Bangladesh; [email protected] 5 Department of Integrative Food, Bioscience and Biotechnology, Chonnam National University, Gwangju 61186, Korea; [email protected] 6 Department of Horticulture, Sher-e-Bangla Agricultural University, Dhaka 1207, Bangladesh; [email protected] 7 Biotechnology Research Institute, Universiti Malaysia Sabah, Jln UMS, Kota Kinabalu 88400, Malaysia * Correspondence: shafi[email protected]; Tel.: +60-14929-4481 Abstract: Protecting food crops from viral pathogens is a significant challenge for agriculture. An integral approach to genome-editing, known as CRISPR/Cas9 (clustered regularly interspaced short palindromic repeats and CRISPR associated protein 9), is used to produce virus-resistant Citation: Shahriar, S.A.; Islam, M.N.; cultivars. The CRISPR/Cas9 tool is an essential part of modern plant breeding due to its attractive Chun, C.N.W.; Rahim, M.A.; Paul, features. Advances in plant breeding programs due to the incorporation of Cas9 have enabled the N.C.; Uddain, J.; Siddiquee, S. -

Complete Nucleotide Sequence and Genetic Organization of Sunflower Mild Mosaic Virus (Summov)
Complete nucleotide sequence and genetic organization of Sunflower mild mosaic virus (SuMMoV) Fabián Giolitti1, Soledad de Breuil1, Nicolas Bejerman1 and Sergio Lenardon1,2 [email protected]; [email protected] 1- Instituto de Patología Vegetal (IPAVE), Centro de Investigaciones Agropecuarias (CIAP), Instituto Nacional de Tecnología Agropecuaria (INTA), Camino 60 cuadras Km. 5,5, X5020ICA, Córdoba, Argentina. 2- Facultad de Agronomía y Veterinaria Universidad, Nacional de Río Cuarto. ABSTRACT Two potyviruses that infect sunflower (Helianthus annuus L.) crops have been reported in Argentina. Sunflower chlorotic mottle virus (SuCMoV) is the most widely distributed one, with two strains: common and ring spot, which have been completely characterized. Sunflower mild mosaic virus (SuMMoV) that produces a chlorotic mild mosaic on sunflower commercial crops was detected in the rural area of Paraná (Entre Ríos Province). This virus has been biologically and serologically characterized but not fully characterized at the molecular level. The aim of this work was to determine the complete sequence of SuMMoV and study its genomic organization. A total RNA extraction was made from a preparation enriched with this virus. The quality of preparation was confirmed through electron microscopy and RT-PCR with specific primers for capsid protein gene. Total RNA obtained was pyrosequenced and the sequences obtained were analyzed. Electron microscopy from rapid preparations of samples enriched with the virus showed high concentration of potyvirus-like particles. RT-PCR showed an aplicon of expected size. The pyrosequencing resulted in a large contig of 9686 nucleotides (nt) with a single open reading frame (ORF) of 3077 amino acids (aa). -

Mosaic Disease of Maize Caused by Sugarcane Mosaic Potyvirus in Sulawesi
56Indonesian Journal of Agricultural Science 2(2) 2001: 56-59 W. Wakman et al. MOSAIC DISEASE OF MAIZE CAUSED BY SUGARCANE MOSAIC POTYVIRUS IN SULAWESI W. Wakmana, M.S. Kontonga, A. Muisa, D.M. Persleyb, and D.S. Teaklec aResearch Institute for Maize and Other Cereals, Jalan Ratulangi, Maros 90154, Indonesia bDepartment of Primary Industries, Indooroopily, Queensland, Australia cDepartment of Microbiology, University of Queensland, Australia ABSTRACT 1978), Brazil (Fernandes and Schaffert, 1978), and Australia (Teakle and Grylls, 1973). In Sulawesi, Mosaic disease of maize and grasses is commonly found in mosaic symptoms were observed in different places, Sulawesi. The symptoms resemble the common mosaic symptoms i.e., Maros, Barru, Bontobili (Gowa), Bantaeng, of virus infection, but the pathogen has not been identified. Bulukumba, and Manado (Wakman and Kontong, The objective of this study was to identify the causal agent of the mosaic disease of maize and grasses in Sulawesi. 1997). Leaf chlorotic disease of Rottboellia sp. (ichy Transmissions of the virus were studied by mechanical grass) found in Bontobili Experimental Farm in 1997 inoculation and the insect vector aphid. Serological study was was similar to maize mosaic with respect to done by using enzyme linked immunosorbent assay (ELISA). transmission into sweet corn seedlings, symptoms, Results of mechanical inoculation showed that the disease was and host range. The objective of this study was to caused by a virus which was transmitted from diseased maize and identify the causal agent of mosaic disease affecting grasses to healthy sweet corn seedlings. The disease was also transmitted by aphid (Rhopalosiphum maidis). Serological maize and grasses.