Mediterranean Pelagic Habitat Oceanographic and Biological Processes, an Overview Maurizio Würtz
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Diversity and Community Structure of Pelagic Cnidarians in the Celebes and Sulu Seas, Southeast Asian Tropical Marginal Seas
Deep-Sea Research I 100 (2015) 54–63 Contents lists available at ScienceDirect Deep-Sea Research I journal homepage: www.elsevier.com/locate/dsri Diversity and community structure of pelagic cnidarians in the Celebes and Sulu Seas, southeast Asian tropical marginal seas Mary M. Grossmann a,n, Jun Nishikawa b, Dhugal J. Lindsay c a Okinawa Institute of Science and Technology Graduate University (OIST), Tancha 1919-1, Onna-son, Okinawa 904-0495, Japan b Tokai University, 3-20-1, Orido, Shimizu, Shizuoka 424-8610, Japan c Japan Agency for Marine-Earth Science and Technology (JAMSTEC), Yokosuka 237-0061, Japan article info abstract Article history: The Sulu Sea is a semi-isolated, marginal basin surrounded by high sills that greatly reduce water inflow Received 13 September 2014 at mesopelagic depths. For this reason, the entire water column below 400 m is stable and homogeneous Received in revised form with respect to salinity (ca. 34.00) and temperature (ca. 10 1C). The neighbouring Celebes Sea is more 19 January 2015 open, and highly influenced by Pacific waters at comparable depths. The abundance, diversity, and Accepted 1 February 2015 community structure of pelagic cnidarians was investigated in both seas in February 2000. Cnidarian Available online 19 February 2015 abundance was similar in both sampling locations, but species diversity was lower in the Sulu Sea, Keywords: especially at mesopelagic depths. At the surface, the cnidarian community was similar in both Tropical marginal seas, but, at depth, community structure was dependent first on sampling location Marginal sea and then on depth within each Sea. Cnidarians showed different patterns of dominance at the two Sill sampling locations, with Sulu Sea communities often dominated by species that are rare elsewhere in Pelagic cnidarians fi Community structure the Indo-Paci c. -

Fisheries Centre
Fisheries Centre The University of British Columbia Working Paper Series Working Paper #2015 - 80 Reconstruction of Syria’s fisheries catches from 1950-2010: Signs of overexploitation Aylin Ulman, Adib Saad, Kyrstn Zylich, Daniel Pauly and Dirk Zeller Year: 2015 Email: [email protected] This working paper is made available by the Fisheries Centre, University of British Columbia, Vancouver, BC, V6T 1Z4, Canada. Reconstruction of Syria’s fisheries catches from 1950-2010: Signs of overexploitation Aylin Ulmana, Adib Saadb, Kyrstn Zylicha, Daniel Paulya, Dirk Zellera a Sea Around Us, Fisheries Centre, University of British Columbia, 2202 Main Mall, Vancouver, BC, V6T 1Z4, Canada b President of Syrian National Committee for Oceanography, Tishreen University, Faculty of Agriculture, P.O. BOX; 1408, Lattakia, Syria [email protected] (corresponding author); [email protected]; [email protected]; [email protected]; [email protected] ABSTRACT Syria’s total marine fisheries catches were estimated for the 1950-2010 time period using a reconstruction approach which accounted for all fisheries removals, including unreported commercial landings, discards, and recreational and subsistence catches. All unreported estimates were added to the official data, as reported by the Syrian Arab Republic to the United Nation’s Food and Agriculture Organization (FAO). Total reconstructed catch for 1950-2010 was around 170,000 t, which is 78% more than the amount reported by Syria to the FAO as their national catch. The unreported components added over 74,000 t of unreported catches, of which 38,600 t were artisanal landings, 16,000 t industrial landings, over 4,000 t recreational catches, 3,000 t subsistence catches and around 12,000 t were discards. -

Mediterranean Marine Science
Mediterranean Marine Science Vol. 13, 2012 First record of Aequorea globosa Eschscholtz, 1829 (Cnidaria: Hydrozoa) in the coast of Syria MAMISH S. Atomic Energy Commission of Syria, Department of Protection and Safety, P.O. Box 6091, Damascus DURUGHAM H. Tishreen University, High Institute of Marine Research, Department of Marine Biology, Lattakia SAID AL MASRI M. Atomic Energy Commission of Syria, Department of Protection and Safety, P.O. Box 6091, Damascus https://doi.org/10.12681/mms.306 Copyright © 2012 To cite this article: MAMISH, S., DURUGHAM, H., & SAID AL MASRI, M. (2012). First record of Aequorea globosa Eschscholtz, 1829 (Cnidaria: Hydrozoa) in the coast of Syria. Mediterranean Marine Science, 13(2), 259-261. doi:https://doi.org/10.12681/mms.306 http://epublishing.ekt.gr | e-Publisher: EKT | Downloaded at 21/02/2020 06:58:00 | Short Communication Mediterranean Marine Science Indexed in WoS (Web of Science, ISI Thomson) and SCOPUS The journal is available on line at http://www.medit-mar-sc.net First record of Aequorea globosa Eschscholtz, 1829 (Cnidaria: Hydrozoa) in the coast of Syria S. MAMISH1, H. DURGHAM2 and M. SAID AL-MASRI1 1 Atomic Energy Commission of Syria, Department of Protection and Safety, P.O. Box 6091, Damascus, Syria 2 Tishreen University, High Institute of Marine Research, Department of Marine Biology, Lattakia, Syria Corresponding author: [email protected] Received: 21 June 2012; Accepted: 02 August 2012; Published on line: 7 September 2012 Abstract The Indo-Pacific jellyfish Aequorea globosa Eschscholtz, 1829 was reported last year for the first time in the Mediterranean Sea from Iskenderun Bay (S. -

CHECKLIST and BIOGEOGRAPHY of FISHES from GUADALUPE ISLAND, WESTERN MEXICO Héctor Reyes-Bonilla, Arturo Ayala-Bocos, Luis E
ReyeS-BONIllA eT Al: CheCklIST AND BIOgeOgRAphy Of fISheS fROm gUADAlUpe ISlAND CalCOfI Rep., Vol. 51, 2010 CHECKLIST AND BIOGEOGRAPHY OF FISHES FROM GUADALUPE ISLAND, WESTERN MEXICO Héctor REyES-BONILLA, Arturo AyALA-BOCOS, LUIS E. Calderon-AGUILERA SAúL GONzáLEz-Romero, ISRAEL SáNCHEz-ALCántara Centro de Investigación Científica y de Educación Superior de Ensenada AND MARIANA Walther MENDOzA Carretera Tijuana - Ensenada # 3918, zona Playitas, C.P. 22860 Universidad Autónoma de Baja California Sur Ensenada, B.C., México Departamento de Biología Marina Tel: +52 646 1750500, ext. 25257; Fax: +52 646 Apartado postal 19-B, CP 23080 [email protected] La Paz, B.C.S., México. Tel: (612) 123-8800, ext. 4160; Fax: (612) 123-8819 NADIA C. Olivares-BAñUELOS [email protected] Reserva de la Biosfera Isla Guadalupe Comisión Nacional de áreas Naturales Protegidas yULIANA R. BEDOLLA-GUzMáN AND Avenida del Puerto 375, local 30 Arturo RAMíREz-VALDEz Fraccionamiento Playas de Ensenada, C.P. 22880 Universidad Autónoma de Baja California Ensenada, B.C., México Facultad de Ciencias Marinas, Instituto de Investigaciones Oceanológicas Universidad Autónoma de Baja California, Carr. Tijuana-Ensenada km. 107, Apartado postal 453, C.P. 22890 Ensenada, B.C., México ABSTRACT recognized the biological and ecological significance of Guadalupe Island, off Baja California, México, is Guadalupe Island, and declared it a Biosphere Reserve an important fishing area which also harbors high (SEMARNAT 2005). marine biodiversity. Based on field data, literature Guadalupe Island is isolated, far away from the main- reviews, and scientific collection records, we pres- land and has limited logistic facilities to conduct scien- ent a comprehensive checklist of the local fish fauna, tific studies. -

Early Stages of Fishes in the Western North Atlantic Ocean Volume
ISBN 0-9689167-4-x Early Stages of Fishes in the Western North Atlantic Ocean (Davis Strait, Southern Greenland and Flemish Cap to Cape Hatteras) Volume One Acipenseriformes through Syngnathiformes Michael P. Fahay ii Early Stages of Fishes in the Western North Atlantic Ocean iii Dedication This monograph is dedicated to those highly skilled larval fish illustrators whose talents and efforts have greatly facilitated the study of fish ontogeny. The works of many of those fine illustrators grace these pages. iv Early Stages of Fishes in the Western North Atlantic Ocean v Preface The contents of this monograph are a revision and update of an earlier atlas describing the eggs and larvae of western Atlantic marine fishes occurring between the Scotian Shelf and Cape Hatteras, North Carolina (Fahay, 1983). The three-fold increase in the total num- ber of species covered in the current compilation is the result of both a larger study area and a recent increase in published ontogenetic studies of fishes by many authors and students of the morphology of early stages of marine fishes. It is a tribute to the efforts of those authors that the ontogeny of greater than 70% of species known from the western North Atlantic Ocean is now well described. Michael Fahay 241 Sabino Road West Bath, Maine 04530 U.S.A. vi Acknowledgements I greatly appreciate the help provided by a number of very knowledgeable friends and colleagues dur- ing the preparation of this monograph. Jon Hare undertook a painstakingly critical review of the entire monograph, corrected omissions, inconsistencies, and errors of fact, and made suggestions which markedly improved its organization and presentation. -

Active Faulting Geometry and Stress
Tectonics RESEARCH ARTICLE Active Faulting Geometry and Stress Pattern Near Complex 10.1029/2018TC004983 Strike-Slip Systems Along the Maghreb Region: Special Section: Constraints on Active Convergence Geodynamics, Crustal and Lithospheric Tectonics, and in the Western Mediterranean active deformation in the Mediterranean Regions Abdelkader Soumaya1,2 , Noureddine Ben Ayed3, Mojtaba Rajabi4 , Mustapha Meghraoui5, (A tribute to Prof. Renato Damien Delvaux6, Ali Kadri3, Moritz Ziegler7,8 , Said Maouche9, and Ahmed Braham2 Funiciello) 1Faculty of Sciences Tunis, University of Manar, Tunis, Tunisia, 2National Office of Mines, Tunisia, 3Faculty of Sciences Bizerte, 4 Key Points: University of Carthage, Tunis, Tunisia, Australian School of Petroleum, University of Adelaide, Adelaide, South Australia, • Maghreb region is characterized by Australia, 5Institut de Physique du Globe de Strasbourg (UMR 7516), Strasbourg, France, 6Earth Sciences Department, Royal different geometries of active Museum for Central Africa, Tervuren, Belgium, 7Helmholtz Centre Potsdam, German Research Centre for Geosciences GFZ, strike-slip faults Potsdam, Germany, 8Institute of Earth and Environmental Science, University of Potsdam, Potsdam, Germany, 9CRAAG, • Present-day active contraction on western Africa-Eurasia boundary is Bouzareah-Alger, Algeria accommodated by a combination of strike-slip and thrust faulting • Second-order tectonic regime across Abstract The Maghreb region (from Tunisia to Gibraltar) is a key area in the western Mediterranean to Maghreb varies with clockwise study the active tectonics and stress pattern across the Africa-Eurasia convergent plate boundary. In the rotation of S from east to west Hmax present study, we compile comprehensive data set of well-constrained crustal stress indicators (from single focal mechanism solutions, formal inversion of focal mechanism solutions, and young geologic fault slip data) based on our and published data analyses. -

The Evolution of Siphonophore Tentilla for Specialized Prey Capture in the Open Ocean
The evolution of siphonophore tentilla for specialized prey capture in the open ocean Alejandro Damian-Serranoa,1, Steven H. D. Haddockb,c, and Casey W. Dunna aDepartment of Ecology and Evolutionary Biology, Yale University, New Haven, CT 06520; bResearch Division, Monterey Bay Aquarium Research Institute, Moss Landing, CA 95039; and cEcology and Evolutionary Biology, University of California, Santa Cruz, CA 95064 Edited by Jeremy B. C. Jackson, American Museum of Natural History, New York, NY, and approved December 11, 2020 (received for review April 7, 2020) Predator specialization has often been considered an evolutionary makes them an ideal system to study the relationships between “dead end” due to the constraints associated with the evolution of functional traits and prey specialization. Like a head of coral, a si- morphological and functional optimizations throughout the organ- phonophore is a colony bearing many feeding polyps (Fig. 1). Each ism. However, in some predators, these changes are localized in sep- feeding polyp has a single tentacle, which branches into a series of arate structures dedicated to prey capture. One of the most extreme tentilla. Like other cnidarians, siphonophores capture prey with cases of this modularity can be observed in siphonophores, a clade of nematocysts, harpoon-like stinging capsules borne within special- pelagic colonial cnidarians that use tentilla (tentacle side branches ized cells known as cnidocytes. Unlike the prey-capture apparatus of armed with nematocysts) exclusively for prey capture. Here we study most other cnidarians, siphonophore tentacles carry their cnidocytes how siphonophore specialists and generalists evolve, and what mor- in extremely complex and organized batteries (3), which are located phological changes are associated with these transitions. -

The Cephalopoda
Carl Chun THE CEPHALOPO PART I: OEGOPSIDA PART II: MYOPSIDA, OCTOPODA ATLAS Carl Chun THE CEPHALOPODA NOTE TO PLATE LXVIII Figure 7 should read Figure 8 Figure 9 should read Figure 7 GERMAN DEEPSEA EXPEDITION 1898-1899. VOL. XVIII SCIENTIFIC RESULTS QF THE GERMAN DEEPSEA EXPEDITION ON BOARD THE*STEAMSHIP "VALDIVIA" 1898-1899 Volume Eighteen UNDER THE AUSPICES OF THE GERMAN MINISTRY OF THE INTERIOR Supervised by CARL CHUN, Director of the Expedition Professor of Zoology , Leipzig. After 1914 continued by AUGUST BRAUER Professor of Zoology, Berlin Carl Chun THE CEPHALOPODA PART I: OEGOPSIDA PART II: MYOPSIDA, OCTOPODA ATLAS Translatedfrom the German ISRAEL PROGRAM FOR SCIENTIFIC TRANSLATIONS Jerusalem 1975 TT 69-55057/2 Published Pursuant to an Agreement with THE SMITHSONIAN INSTITUTION and THE NATIONAL SCIENCE FOUNDATION, WASHINGTON, D.C. Since the study of the Cephalopoda is a very specialized field with a unique and specific terminology and phrase- ology, it was necessary to edit the translation in a technical sense to insure that as accurate and meaningful a represen- tation of Chun's original work as possible would be achieved. We hope to have accomplished this responsibility. Clyde F. E. Roper and Ingrid H. Roper Technical Editors Copyright © 1975 Keter Publishing House Jerusalem Ltd. IPST Cat. No. 05452 8 ISBN 7065 1260 X Translated by Albert Mercado Edited by Prof. O. Theodor Copy-edited by Ora Ashdit Composed, Printed and Bound by Keterpress Enterprises, Jerusalem, Israel Available from the U. S. DEPARTMENT OF COMMERCE National Technical Information Service Springfield, Va. 22151 List of Plates I Thaumatolampas diadema of luminous o.rgans 95 luminous organ 145 n.gen.n.sp. -

Africa-Arabia-Eurasia Plate Interactions and Implications for the Dynamics of Mediterranean Subduction and Red Sea Rifting
This page added by the GeoPRISMS office. Africa-Arabia-Eurasia plate interactions and implications for the dynamics of Mediterranean subduction and Red Sea rifting Authors: R. Reilinger, B. Hager, L. Royden, C. Burchfiel, R. Van der Hilst Department of Earth, Atmospheric, and Planetary Sciences, Massachusetts Institute of Technology, Cambridge, MA USA, [email protected], Tel: (617)253 -7860 This page added by the GeoPRISMS office. Our proposed GeoPRISMS Initiative is based on the premise that understanding the mechanics of plate motions (i.e., the force balance on the plates) is necessary to develop realistic models for plate interactions, including processes at subduction and extensional (rifting) plate boundaries. Important advances are being made with new geologic and geophysical techniques and observations that are providing fundamental insights into the dynamics of these plate tectonic processes. Our proposed research addresses directly the following questions identified in the GeoPRISMS SCD Draft Science Plan: 4.2 (How does deformation across the subduction plate boundary evolve in space and time, through the seismic cycle and beyond?), 4.6 (What are the physical and chemical conditions that control subduction zone initiation and the development of mature arc systems?), and 4.7 (What are the critical feedbacks between surface processes and subduction zone mechanics and dynamics?). It has long been recognized that the Greater Mediterranean region provides a natural laboratory to study a wide range of geodynamic processes (Figure 1) including ocean subduction and continent- continent collision (Hellenic arc, Arabia-Eurasia collision), lithospheric delamination (E Turkey High Plateau, Alboran Sea/High Atlas), back-arc extension (Mediterranean basins, including Alboran, Central Mediterranean, Aegean), “escape” tectonics and associated continental transform faulting (Anatolia, North and East Anatolian faults), and active continental and ocean rifting (East African and northern Red Sea rifting, central Red Sea and Gulf of Aden young ocean rifting). -

Aspects of the Natural History of Pelagic Cephalopods of the Hawaiian Mesopelagic-Boundary Region 1
Pacific Science (1995), vol. 49, no. 2: 143-155 © 1995 by University of Hawai'i Press. All rights reserved Aspects of the Natural History of Pelagic Cephalopods of the Hawaiian Mesopelagic-Boundary Region 1 RICHARD EDWARD YOUNG 2 ABSTRACT: Pelagic cephalopods of the mesopelagic-boundary region in Hawai'i have proven difficult to sample but seem to occupy a variety ofhabitats within this zone. Abralia trigonura Berry inhabits the zone only as adults; A. astrosticta Berry may inhabit the inner boundary zone, and Pterygioteuthis giardi Fischer appears to be a facultative inhabitant. Three other mesopelagic species, Liocranchia reinhardti (Steenstrup), Chiroteuthis imperator Chun, and Iridoteuthis iris (Berry), are probable inhabitants; the latter two are suspected to be nonvertical migrants. The mesopelagic-boundary region also contains a variety of other pelagic cephalopods. Some are transients, common species of the mesopelagic zone in offshore waters such as Abraliopsis spp., neritic species such as Euprymna scolopes Berry, and oceanic epipelagic species such as Tremoctopus violaceus Chiaie and Argonauta argo Linnaeus. Others are appar ently permanent but either epipelagic (Onychoteuthis sp. C) or demersal (No totodarus hawaiiensis [Berry] and Haliphron atlanticus Steenstrup). Submersible observations show that Nototodarus hawaiiensis commonly "sits" on the bot tom and Haliphron atlanticus broods its young in the manner of some pelagic octopods. THE CONCEPT OF the mesopelagic-boundary over bottom depths of the same range. The region (m-b region) was first introduced by designation of an inner zone is based on Reid et al. (1991), although a general asso Reid'sfinding mesopelagic fishes resident there ciation of various mesopelagic animals with during both the day and night; mesopelagic land masses has been known for some time. -

Marine Mammals and Sea Turtles of the Mediterranean and Black Seas
Marine mammals and sea turtles of the Mediterranean and Black Seas MEDITERRANEAN AND BLACK SEA BASINS Main seas, straits and gulfs in the Mediterranean and Black Sea basins, together with locations mentioned in the text for the distribution of marine mammals and sea turtles Ukraine Russia SEA OF AZOV Kerch Strait Crimea Romania Georgia Slovenia France Croatia BLACK SEA Bosnia & Herzegovina Bulgaria Monaco Bosphorus LIGURIAN SEA Montenegro Strait Pelagos Sanctuary Gulf of Italy Lion ADRIATIC SEA Albania Corsica Drini Bay Spain Dardanelles Strait Greece BALEARIC SEA Turkey Sardinia Algerian- TYRRHENIAN SEA AEGEAN SEA Balearic Islands Provençal IONIAN SEA Syria Basin Strait of Sicily Cyprus Strait of Sicily Gibraltar ALBORAN SEA Hellenic Trench Lebanon Tunisia Malta LEVANTINE SEA Israel Algeria West Morocco Bank Tunisian Plateau/Gulf of SirteMEDITERRANEAN SEA Gaza Strip Jordan Suez Canal Egypt Gulf of Sirte Libya RED SEA Marine mammals and sea turtles of the Mediterranean and Black Seas Compiled by María del Mar Otero and Michela Conigliaro The designation of geographical entities in this book, and the presentation of the material, do not imply the expression of any opinion whatsoever on the part of IUCN concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. The views expressed in this publication do not necessarily reflect those of IUCN. Published by Compiled by María del Mar Otero IUCN Centre for Mediterranean Cooperation, Spain © IUCN, Gland, Switzerland, and Malaga, Spain Michela Conigliaro IUCN Centre for Mediterranean Cooperation, Spain Copyright © 2012 International Union for Conservation of Nature and Natural Resources With the support of Catherine Numa IUCN Centre for Mediterranean Cooperation, Spain Annabelle Cuttelod IUCN Species Programme, United Kingdom Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holder provided the sources are fully acknowledged. -
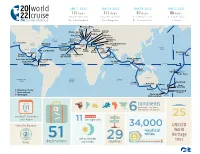
World Cruise - 2022 Use the Down Arrow from a Form Field
This document contains both information and form fields. To read information, World Cruise - 2022 use the Down Arrow from a form field. 20 world JAN 5, 2022 JAN 19, 2022 JAN 19, 2022 JAN 5, 2022 111 days 111 days 97 days 88 days 22 cruise roundtrip from roundtrip from Los Angeles to Ft. Lauderdale Ft. Lauderdale Los Angeles Ft. Lauderdale to Rome Florence/Pisa (Livorno) Genoa Rome (Civitavecchia) Catania Monte Carlo (Sicily) MONACO ITALY Naples Marseille Mykonos FRANCE GREECE Kusadasi PORTUGAL Atlantic Barcelona Heraklion Ocean SPAIN (Crete) Los Angeles Lisbon TURKEY UNITED Bermuda Ceuta Jerusalem/Bethlehem STATES (West End) (Spanish Morocco) Seville (Ashdod) ine (Cadiz) ISRAEL Athens e JORDAN Dubai Agadir (Piraeus) Aqaba Pacific MEXICO Madeira UNITED ARAB Ocean MOROCCO l Dat L (Funchal) Malta EMIRATES Ft. Lauderdale CANARY (Valletta) Suez Abu ISLANDS Canal Honolulu Huatulco Dhabi ne inn Puerto Santa Cruz Lanzarote OMAN a a Hawaii r o Hilo Vallarta NICARAGUA (Arrecife) de Tenerife Salãlah t t Kuala Lumpur I San Juan del Sur Cartagena (Port Kelang) Costa Rica COLOMBIA Sri Lanka PANAMA (Puntarenas) Equator (Colombo) Singapore Equator Panama Canal MALAYSIA INDONESIA Bali SAMOA (Benoa) AMERICAN Apia SAMOA Pago Pago AUSTRALIA South Pacific South Indian Ocean Atlantic Ocean Ocean Perth Auckland (Fremantle) Adelaide Sydney New Plymouth Burnie Picton Departure Ports Tasmania Christchurch More Ashore (Lyttelton) Overnight Fiordland NEW National Park ZEALAND up to continentscontinents (North America, South America, 111 51 Australia, Europe, Africa