Alpha-Decay Studies in the Heavy-Element Region
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Table 2.Iii.1. Fissionable Isotopes1
FISSIONABLE ISOTOPES Charles P. Blair Last revised: 2012 “While several isotopes are theoretically fissionable, RANNSAD defines fissionable isotopes as either uranium-233 or 235; plutonium 238, 239, 240, 241, or 242, or Americium-241. See, Ackerman, Asal, Bale, Blair and Rethemeyer, Anatomizing Radiological and Nuclear Non-State Adversaries: Identifying the Adversary, p. 99-101, footnote #10, TABLE 2.III.1. FISSIONABLE ISOTOPES1 Isotope Availability Possible Fission Bare Critical Weapon-types mass2 Uranium-233 MEDIUM: DOE reportedly stores Gun-type or implosion-type 15 kg more than one metric ton of U- 233.3 Uranium-235 HIGH: As of 2007, 1700 metric Gun-type or implosion-type 50 kg tons of HEU existed globally, in both civilian and military stocks.4 Plutonium- HIGH: A separated global stock of Implosion 10 kg 238 plutonium, both civilian and military, of over 500 tons.5 Implosion 10 kg Plutonium- Produced in military and civilian 239 reactor fuels. Typically, reactor Plutonium- grade plutonium (RGP) consists Implosion 40 kg 240 of roughly 60 percent plutonium- Plutonium- 239, 25 percent plutonium-240, Implosion 10-13 kg nine percent plutonium-241, five 241 percent plutonium-242 and one Plutonium- percent plutonium-2386 (these Implosion 89 -100 kg 242 percentages are influenced by how long the fuel is irradiated in the reactor).7 1 This table is drawn, in part, from Charles P. Blair, “Jihadists and Nuclear Weapons,” in Gary A. Ackerman and Jeremy Tamsett, ed., Jihadists and Weapons of Mass Destruction: A Growing Threat (New York: Taylor and Francis, 2009), pp. 196-197. See also, David Albright N 2 “Bare critical mass” refers to the absence of an initiator or a reflector. -

The Development of the Periodic Table and Its Consequences Citation: J
Firenze University Press www.fupress.com/substantia The Development of the Periodic Table and its Consequences Citation: J. Emsley (2019) The Devel- opment of the Periodic Table and its Consequences. Substantia 3(2) Suppl. 5: 15-27. doi: 10.13128/Substantia-297 John Emsley Copyright: © 2019 J. Emsley. This is Alameda Lodge, 23a Alameda Road, Ampthill, MK45 2LA, UK an open access, peer-reviewed article E-mail: [email protected] published by Firenze University Press (http://www.fupress.com/substantia) and distributed under the terms of the Abstract. Chemistry is fortunate among the sciences in having an icon that is instant- Creative Commons Attribution License, ly recognisable around the world: the periodic table. The United Nations has deemed which permits unrestricted use, distri- 2019 to be the International Year of the Periodic Table, in commemoration of the 150th bution, and reproduction in any medi- anniversary of the first paper in which it appeared. That had been written by a Russian um, provided the original author and chemist, Dmitri Mendeleev, and was published in May 1869. Since then, there have source are credited. been many versions of the table, but one format has come to be the most widely used Data Availability Statement: All rel- and is to be seen everywhere. The route to this preferred form of the table makes an evant data are within the paper and its interesting story. Supporting Information files. Keywords. Periodic table, Mendeleev, Newlands, Deming, Seaborg. Competing Interests: The Author(s) declare(s) no conflict of interest. INTRODUCTION There are hundreds of periodic tables but the one that is widely repro- duced has the approval of the International Union of Pure and Applied Chemistry (IUPAC) and is shown in Fig.1. -

A Measurement of the 2 Neutrino Double Beta Decay Rate of 130Te in the CUORICINO Experiment by Laura Katherine Kogler
A measurement of the 2 neutrino double beta decay rate of 130Te in the CUORICINO experiment by Laura Katherine Kogler A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Physics in the Graduate Division of the University of California, Berkeley Committee in charge: Professor Stuart J. Freedman, Chair Professor Yury G. Kolomensky Professor Eric B. Norman Fall 2011 A measurement of the 2 neutrino double beta decay rate of 130Te in the CUORICINO experiment Copyright 2011 by Laura Katherine Kogler 1 Abstract A measurement of the 2 neutrino double beta decay rate of 130Te in the CUORICINO experiment by Laura Katherine Kogler Doctor of Philosophy in Physics University of California, Berkeley Professor Stuart J. Freedman, Chair CUORICINO was a cryogenic bolometer experiment designed to search for neutrinoless double beta decay and other rare processes, including double beta decay with two neutrinos (2νββ). The experiment was located at Laboratori Nazionali del Gran Sasso and ran for a period of about 5 years, from 2003 to 2008. The detector consisted of an array of 62 TeO2 crystals arranged in a tower and operated at a temperature of ∼10 mK. Events depositing energy in the detectors, such as radioactive decays or impinging particles, produced thermal pulses in the crystals which were read out using sensitive thermistors. The experiment included 4 enriched crystals, 2 enriched with 130Te and 2 with 128Te, in order to aid in the measurement of the 2νββ rate. The enriched crystals contained a total of ∼350 g 130Te. The 128-enriched (130-depleted) crystals were used as background monitors, so that the shared backgrounds could be subtracted from the energy spectrum of the 130- enriched crystals. -

Lecture 22 Alpha Decay 1 Introduction 2 Fission and Fusion
Nuclear and Particle Physics - Lecture 22 Alpha decay 1 Introduction We have looked at gamma decays (due to the EM force) and beta decays (due to the weak force) and now will look at alpha decays, which are due to the strong/nuclear force. In contrast to the previous decays which do not change A, alpha decays happen by emission of some of the 4 nucleons from the nucleus. Specifically, for alpha decay, an alpha particle, 2He, is ejected so generically A A−4 4 X − Y + He Z !Z 2 2 for some X and Y . Y clearly has a different number of nucleons to X. Compare how alpha and beta decays move the nuclei around in Z,N plane N β+ β- α Z Note that gamma decays cannot change Z, N or A. 2 Fission and fusion Alpha decay is in fact only one specific case of a whole range of processes which involve emission of nucleons. These range from single proton or neutron emission up to splitting the nucleus into two roughly equal parts. Particularly in the latter case, these are called fission decays. Which nuclei would we expect to fission? The binding energy per nucleon curve shows that 56 the maximum occurs around 26Fe and drops off to either side. Hence, both small A and large A nuclei are less strongly bound per nucleon than medium A. This means there will be some energy release if two small nuclei are combined into a larger one, a process called fusion which will be discussed later in the course. -

Radioactive Decay
North Berwick High School Department of Physics Higher Physics Unit 2 Particles and Waves Section 3 Fission and Fusion Section 3 Fission and Fusion Note Making Make a dictionary with the meanings of any new words. Einstein and nuclear energy 1. Write down Einstein’s famous equation along with units. 2. Explain the importance of this equation and its relevance to nuclear power. A basic model of the atom 1. Copy the components of the atom diagram and state the meanings of A and Z. 2. Copy the table on page 5 and state the difference between elements and isotopes. Radioactive decay 1. Explain what is meant by radioactive decay and copy the summary table for the three types of nuclear radiation. 2. Describe an alpha particle, including the reason for its short range and copy the panel showing Plutonium decay. 3. Describe a beta particle, including its range and copy the panel showing Tritium decay. 4. Describe a gamma ray, including its range. Fission: spontaneous decay and nuclear bombardment 1. Describe the differences between the two methods of decay and copy the equation on page 10. Nuclear fission and E = mc2 1. Explain what is meant by the terms ‘mass difference’ and ‘chain reaction’. 2. Copy the example showing the energy released during a fission reaction. 3. Briefly describe controlled fission in a nuclear reactor. Nuclear fusion: energy of the future? 1. Explain why nuclear fusion might be a preferred source of energy in the future. 2. Describe some of the difficulties associated with maintaining a controlled fusion reaction. -
![Arxiv:1901.01410V3 [Astro-Ph.HE] 1 Feb 2021 Mental Information Is Available, and One Has to Rely Strongly on Theoretical Predictions for Nuclear Properties](https://docslib.b-cdn.net/cover/8159/arxiv-1901-01410v3-astro-ph-he-1-feb-2021-mental-information-is-available-and-one-has-to-rely-strongly-on-theoretical-predictions-for-nuclear-properties-508159.webp)
Arxiv:1901.01410V3 [Astro-Ph.HE] 1 Feb 2021 Mental Information Is Available, and One Has to Rely Strongly on Theoretical Predictions for Nuclear Properties
Origin of the heaviest elements: The rapid neutron-capture process John J. Cowan∗ HLD Department of Physics and Astronomy, University of Oklahoma, 440 W. Brooks St., Norman, OK 73019, USA Christopher Snedeny Department of Astronomy, University of Texas, 2515 Speedway, Austin, TX 78712-1205, USA James E. Lawlerz Physics Department, University of Wisconsin-Madison, 1150 University Avenue, Madison, WI 53706-1390, USA Ani Aprahamianx and Michael Wiescher{ Department of Physics and Joint Institute for Nuclear Astrophysics, University of Notre Dame, 225 Nieuwland Science Hall, Notre Dame, IN 46556, USA Karlheinz Langanke∗∗ GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany and Institut f¨urKernphysik (Theoriezentrum), Fachbereich Physik, Technische Universit¨atDarmstadt, Schlossgartenstraße 2, 64298 Darmstadt, Germany Gabriel Mart´ınez-Pinedoyy GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany; Institut f¨urKernphysik (Theoriezentrum), Fachbereich Physik, Technische Universit¨atDarmstadt, Schlossgartenstraße 2, 64298 Darmstadt, Germany; and Helmholtz Forschungsakademie Hessen f¨urFAIR, GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany Friedrich-Karl Thielemannzz Department of Physics, University of Basel, Klingelbergstrasse 82, 4056 Basel, Switzerland and GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany (Dated: February 2, 2021) The production of about half of the heavy elements found in nature is assigned to a spe- cific astrophysical nucleosynthesis process: the rapid neutron capture process (r-process). Although this idea has been postulated more than six decades ago, the full understand- ing faces two types of uncertainties/open questions: (a) The nucleosynthesis path in the nuclear chart runs close to the neutron-drip line, where presently only limited experi- arXiv:1901.01410v3 [astro-ph.HE] 1 Feb 2021 mental information is available, and one has to rely strongly on theoretical predictions for nuclear properties. -

Recent Developments in Radioactive Charged-Particle Emissions
Recent developments in radioactive charged-particle emissions and related phenomena Chong Qi, Roberto Liotta, Ramon Wyss Department of Physics, Royal Institute of Technology (KTH), SE-10691 Stockholm, Sweden October 19, 2018 Abstract The advent and intensive use of new detector technologies as well as radioactive ion beam facilities have opened up possibilities to investigate alpha, proton and cluster decays of highly unstable nuclei. This article provides a review of the current status of our understanding of clustering and the corresponding radioactive particle decay process in atomic nuclei. We put alpha decay in the context of charged-particle emissions which also include one- and two-proton emissions as well as heavy cluster decay. The experimental as well as the theoretical advances achieved recently in these fields are presented. Emphasis is given to the recent discoveries of charged-particle decays from proton-rich nuclei around the proton drip line. Those decay measurements have shown to provide an important probe for studying the structure of the nuclei involved. Developments on the theoretical side in nuclear many-body theories and supercomputing facilities have also made substantial progress, enabling one to study the nuclear clusterization and decays within a microscopic and consistent framework. We report on properties induced by the nuclear interaction acting in the nuclear medium, like the pairing interaction, which have been uncovered by studying the microscopic structure of clusters. The competition between cluster formations as compared to the corresponding alpha-particle formation are included. In the review we also describe the search for super-heavy nuclei connected by chains of alpha and other radioactive particle decays. -

Rapid Separations of Protactinium and Uranium Radioisotopes From
754 LETTERS TO THE EDITOR may be as small as one micron in diameter. Such particles are solutions with ether were required to further eliminate non products of breakdown or are introduced mto the enclosed system volatile foreign material which would extract into the ether. at the time of assembly. If special precautions were not taken to Ignition of the ether sample gave a weightless plate of the uranium eliminate stray particles, the subnormal breakdown strengths isotopes. obtained would lay within ±1O percent of a mean value which Approximate times required for the steps were: solution, for a given method of electrode polishing would be repeatable. extraction, and separation 10-20 seconds; washing 10-15 seconds; These mean values, reported earlier,! are in agreement with the and making of sample plate ("-'1 cc ether) ",15 seconds. results published by other workers. In nuclear chemical work where the main interest is in charac Experiments are continuing to determine whether the maximum terizing particular isotopes, emphasis is on radiochemical purity values reported here are indeed the intrinsic electric strengths of of the isotope rather than on chemical yield. The above procedures the liquids concerned. give only a 10-20 percent chemical yield-fa of which can be The author is indebted to the National Research Council of rapidly placed on a plate for counting, These yields are quite Canada for the award of a Fellowship which enabled him to carry satisfactory for the purpose, however, since contaminating isotopes out this work. have been reduced by a factor of 104 to lOS. Experimental results have shown that about 1 part in 104 of actinium, 1 part in lOS of 1 W. -

Periodic Table 1 Periodic Table
Periodic table 1 Periodic table This article is about the table used in chemistry. For other uses, see Periodic table (disambiguation). The periodic table is a tabular arrangement of the chemical elements, organized on the basis of their atomic numbers (numbers of protons in the nucleus), electron configurations , and recurring chemical properties. Elements are presented in order of increasing atomic number, which is typically listed with the chemical symbol in each box. The standard form of the table consists of a grid of elements laid out in 18 columns and 7 Standard 18-column form of the periodic table. For the color legend, see section Layout, rows, with a double row of elements under the larger table. below that. The table can also be deconstructed into four rectangular blocks: the s-block to the left, the p-block to the right, the d-block in the middle, and the f-block below that. The rows of the table are called periods; the columns are called groups, with some of these having names such as halogens or noble gases. Since, by definition, a periodic table incorporates recurring trends, any such table can be used to derive relationships between the properties of the elements and predict the properties of new, yet to be discovered or synthesized, elements. As a result, a periodic table—whether in the standard form or some other variant—provides a useful framework for analyzing chemical behavior, and such tables are widely used in chemistry and other sciences. Although precursors exist, Dmitri Mendeleev is generally credited with the publication, in 1869, of the first widely recognized periodic table. -

Nuclear Physics
Massachusetts Institute of Technology 22.02 INTRODUCTION to APPLIED NUCLEAR PHYSICS Spring 2012 Prof. Paola Cappellaro Nuclear Science and Engineering Department [This page intentionally blank.] 2 Contents 1 Introduction to Nuclear Physics 5 1.1 Basic Concepts ..................................................... 5 1.1.1 Terminology .................................................. 5 1.1.2 Units, dimensions and physical constants .................................. 6 1.1.3 Nuclear Radius ................................................ 6 1.2 Binding energy and Semi-empirical mass formula .................................. 6 1.2.1 Binding energy ................................................. 6 1.2.2 Semi-empirical mass formula ......................................... 7 1.2.3 Line of Stability in the Chart of nuclides ................................... 9 1.3 Radioactive decay ................................................... 11 1.3.1 Alpha decay ................................................... 11 1.3.2 Beta decay ................................................... 13 1.3.3 Gamma decay ................................................. 15 1.3.4 Spontaneous fission ............................................... 15 1.3.5 Branching Ratios ................................................ 15 2 Introduction to Quantum Mechanics 17 2.1 Laws of Quantum Mechanics ............................................. 17 2.2 States, observables and eigenvalues ......................................... 18 2.2.1 Properties of eigenfunctions ......................................... -
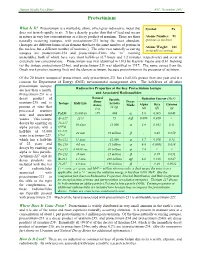
Protactinium
Human Health Fact Sheet ANL, November 2001 Protactinium What Is It? Protactinium is a malleable, shiny, silver-gray radioactive metal that Symbol: Pa does not tarnish rapidly in air. It has a density greater than that of lead and occurs in nature in very low concentrations as a decay product of uranium. There are three Atomic Number: 91 naturally occurring isotopes, with protactinium-231 being the most abundant. (protons in nucleus) (Isotopes are different forms of an element that have the same number of protons in Atomic Weight: 231 the nucleus but a different number of neutrons.) The other two naturally occurring (naturally occurring) isotopes are protactinium-234 and protactinium-234m (the “m” meaning metastable), both of which have very short half-lives (6.7 hours and 1.2 minutes, respectively) and occur in extremely low concentrations. Protactinium was first identified in 1913 by Kasimir Fajans and O.H. Gohring (as the isotope protactinium-234m), and protactinium-231 was identified in 1917. The name comes from the Greek work protos (meaning first) and the element actinium, because protactinium is the precursor of actinium. Of the 20 known isotopes of protactinium, only protactinium-231 has a half-life greater than one year and is a concern for Department of Energy (DOE) environmental management sites. The half-lives of all other protactinium isotopes Radioactive Properties of the Key Protactinium Isotope are less than a month. Protactinium-231 is a and Associated Radionuclides Natural decay product of Specific Radiation Energy (MeV) Abun- Decay uranium-235 and is Isotope Half-Life Activity dance Mode Alpha Beta Gamma present at sites that (Ci/g) (%) (α) (β) (γ) processed uranium α ores and associated Pa231 33,000 yr >99 .048 5.0 0.065 0.048 wastes. -

A Global Scavenging and Circulation Ocean Model of Thorium-230 and Protactinium-231 with Improved Particle Dynamics (NEMO–Prothorp 0.1)
Geosci. Model Dev., 11, 3537–3556, 2018 https://doi.org/10.5194/gmd-11-3537-2018 © Author(s) 2018. This work is distributed under the Creative Commons Attribution 4.0 License. A global scavenging and circulation ocean model of thorium-230 and protactinium-231 with improved particle dynamics (NEMO–ProThorP 0.1) Marco van Hulten1,2, Jean-Claude Dutay1, and Matthieu Roy-Barman1 1Laboratoire des Sciences du Climat et de l’Environnement, IPSL, CEA–Orme des Merisiers, 91191 Gif-sur-Yvette, France 2Geophysical Institute, University of Bergen, Bergen, Norway Correspondence: Marco van Hulten ([email protected]) Received: 1 November 2017 – Discussion started: 8 December 2017 Revised: 16 August 2018 – Accepted: 20 August 2018 – Published: 31 August 2018 Abstract. In this paper we set forth a 3-D ocean model of the can be done based on our model as its source code is readily radioactive trace isotopes 230Th and 231Pa. The interest arises available. from the fact that these isotopes are extensively used for in- vestigating particle transport in the ocean and reconstructing past ocean circulation. The tracers are reversibly scavenged 1 Introduction by biogenic and lithogenic particles. Our simulations of 230Th and 231Pa are based on the Oceanic circulation and the carbon cycle play a major role NEMO–PISCES ocean biogeochemistry general circulation in the regulation of the past and present climate. Heat and model, which includes biogenic particles, namely small and carbon dioxide in the atmosphere tend to equilibrate with the big particulate organic carbon, calcium carbonate and bio- ocean surface and are transported down into the deep ocean genic silica.