Cetopsis Coecutiens) Ecological Risk Screening Summary
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Vampire Fish Rides Giant Catfishes in The
FREE MEALS ON LONG-DISTANCE CRUISERS: THE VAMPIRE FISH RIDES GIANT CATFISHES IN THE AMAZON Jansen Zuanon*, Ivan Sazima** Biota Neotropica v5 (n1) – http://www.biotaneotropica.org.br/v5n1/pt/abstract?article+BN03005012005 Recebido em 16/08/04 - Versão revisada recebida em 21/01/05 - Publicado em 10/02/05. *CPBA, Caixa Postal 478, INPA-Instituto Nacional de Pesquisas da Amazônia,69083-970 Manaus, Amazonas, Brasil **Departamento de Zoologia e Museu de História Natural, Caixa Postal 6109, Universidade Estadual de Campinas, 13083-970 Campinas, São Paulo, Brazil (www.unicamp.br) **Corresponding author. Tel: + 55-19-3788 7292; fax: +55-19-3289 3124; e-mail: [email protected] Abstract - The trichomycterid catfishes known as candirus are renowned for their blood feeding, but information on their habits under natural conditions is very fragmentary and generally restricted to hosts or habitats. We recorded an undescribed species of the vandelliine genus Paracanthopoma riding the giant jau catfish, Zungaro zungaro (Pimelodidae), in the upper Amazon. The candirus were found on the host’s caudal and pectoral fins, as well as the base of the dorsal fin, with their snouts buried up to the eyes in the tough skin of the catfish host. All of them had small amounts of partly digested blood in the distal part of the gut. Along the host’s dorsal fin base we found a few additional tiny holes, most of them healed. We suggest that Paracanthopoma feeds on the gill chamber of its hosts, and that the individuals we found were taking a ride partly buried into the host’s skin. -

Amazon Alive: a Decade of Discoveries 1999-2009
Amazon Alive! A decade of discovery 1999-2009 The Amazon is the planet’s largest rainforest and river basin. It supports countless thousands of species, as well as 30 million people. © Brent Stirton / Getty Images / WWF-UK © Brent Stirton / Getty Images The Amazon is the largest rainforest on Earth. It’s famed for its unrivalled biological diversity, with wildlife that includes jaguars, river dolphins, manatees, giant otters, capybaras, harpy eagles, anacondas and piranhas. The many unique habitats in this globally significant region conceal a wealth of hidden species, which scientists continue to discover at an incredible rate. Between 1999 and 2009, at least 1,200 new species of plants and vertebrates have been discovered in the Amazon biome (see page 6 for a map showing the extent of the region that this spans). The new species include 637 plants, 257 fish, 216 amphibians, 55 reptiles, 16 birds and 39 mammals. In addition, thousands of new invertebrate species have been uncovered. Owing to the sheer number of the latter, these are not covered in detail by this report. This report has tried to be comprehensive in its listing of new plants and vertebrates described from the Amazon biome in the last decade. But for the largest groups of life on Earth, such as invertebrates, such lists do not exist – so the number of new species presented here is no doubt an underestimate. Cover image: Ranitomeya benedicta, new poison frog species © Evan Twomey amazon alive! i a decade of discovery 1999-2009 1 Ahmed Djoghlaf, Executive Secretary, Foreword Convention on Biological Diversity The vital importance of the Amazon rainforest is very basic work on the natural history of the well known. -

Amazon Alive!
Amazon Alive! A decade of discovery 1999-2009 The Amazon is the planet’s largest rainforest and river basin. It supports countless thousands of species, as well as 30 million people. © Brent Stirton / Getty Images / WWF-UK © Brent Stirton / Getty Images The Amazon is the largest rainforest on Earth. It’s famed for its unrivalled biological diversity, with wildlife that includes jaguars, river dolphins, manatees, giant otters, capybaras, harpy eagles, anacondas and piranhas. The many unique habitats in this globally significant region conceal a wealth of hidden species, which scientists continue to discover at an incredible rate. Between 1999 and 2009, at least 1,200 new species of plants and vertebrates have been discovered in the Amazon biome (see page 6 for a map showing the extent of the region that this spans). The new species include 637 plants, 257 fish, 216 amphibians, 55 reptiles, 16 birds and 39 mammals. In addition, thousands of new invertebrate species have been uncovered. Owing to the sheer number of the latter, these are not covered in detail by this report. This report has tried to be comprehensive in its listing of new plants and vertebrates described from the Amazon biome in the last decade. But for the largest groups of life on Earth, such as invertebrates, such lists do not exist – so the number of new species presented here is no doubt an underestimate. Cover image: Ranitomeya benedicta, new poison frog species © Evan Twomey amazon alive! i a decade of discovery 1999-2009 1 Ahmed Djoghlaf, Executive Secretary, Foreword Convention on Biological Diversity The vital importance of the Amazon rainforest is very basic work on the natural history of the well known. -

Permen-Kp/… Tentang Larangan Pemasukan Jenis Ikan Membahayakan Dan Merugikan Ke Dalam Wilayah Negara Republik Indonesia
1 RANCANGAN PERATURAN MENTERI KELAUTAN DAN PERIKANAN REPUBLIK INDONESIA NOMOR …/PERMEN-KP/… TENTANG LARANGAN PEMASUKAN JENIS IKAN MEMBAHAYAKAN DAN MERUGIKAN KE DALAM WILAYAH NEGARA REPUBLIK INDONESIA DENGAN RAHMAT TUHAN YANG MAHA ESA MENTERI KELAUTAN DAN PERIKANAN REPUBLIK INDONESIA, Menimbang : a. bahwa untuk melestarikan sumber daya ikan dan lingkungannya serta untuk mencegah terjadinya hal- hal yang membahayakan dan merugikan masyarakat, pembudidayaan ikan, sumber daya ikan, dan/atau lingkungan sumber daya ikan, perlu mengganti Peraturan Menteri Kelautan dan Perikanan Nomor 41/PERMEN-KP/2014 tentang Larangan Pemasukan Jenis Ikan Berbahaya dari Luar Negeri ke Dalam Wilayah Negara Republik Indonesia; b. bahwa berdasarkan pertimbangan sebagaimana dimaksud dalam huruf a, perlu menetapkan Peraturan Menteri Kelautan dan Perikanan tentang Larangan Pemasukan Jenis Ikan Membahayakan dan Merugikan ke Dalam Wilayah Negara Republik Indonesia; Mengingat : 1. Pasal 17 ayat (3) Undang-Undang Dasar Negara Republik Indonesia Tahun 1945; - 2 - 2. Undang-Undang Nomor 31 Tahun 2004 tentang Perikanan (Lembaran Negara Republik Indonesia Tahun 2004 Nomor 118, Tambahan Lembaran Negara Republik Indonesia Nomor 4433) sebagaimana telah diubah dengan Undang-Undang Nomor 45 Tahun 2009 tentang Perubahan atas Undang-Undang Nomor 31 Tahun 2004 tentang Perikanan (Lembaran Negara Republik Indonesia Tahun 2009 Nomor 154, Tambahan Lembaran Negara Republik Indonesia Nomor 5073); 3. Peraturan Presiden Nomor 63 Tahun 2015 tentang Kementerian Kelautan dan Perikanan (Lembaran Negara Republik Indonesia Tahun 2015 Nomor 111) sebagaimana telah diubah dengan Peraturan Presiden Nomor 2 Tahun 2017 tentang Perubahan atas Peraturan Presiden Nomor 63 Tahun 2015 tentang Kementerian Kelautan dan Perikanan (Lembaran Negara Republik Indonesia Tahun 2017 Nomor 5); 4. Peraturan Presiden Nomor 68 Tahun 2019 tentang Organisasi Kementerian Negara (Lembaran Negara Republik Indonesia Tahun 2019 Nomor 203); 5. -

Systematic Index 881 SYSTEMATIC INDEX
systematic index 881 SYSTEMATIC INDEX Acanthodoras 28, 41, 544, 546-548 Anchoviella sp. 20, 152, 153, 158, 159 Acanthodoras cataphractus 28, 41, 544, 546-548 Ancistrinae 412, 438 ACESTRORHYNCHIDAE 24, 130, 168, 334-337 ANCISTRINI 412, 438 Acestrorhynchus 24, 72, 82, 84, 334-337 Ancistrus 438, 442-449 Acestrorhynchus falcatus 24, 334-336 Ancistrus aff. hoplogenys 26, 443-446 Acestrorhynchus guianensis 336 Ancistrus gr. leucostictus 26, 443, 446, 447 Acestrorhynchus microlepis 24, 82, 84, 334, 336, Ancistrus sp. ‘reticulate’ 26, 443, 446, 447 337 Ancistrus temminckii 26, 443, 448, 449 ACHIRIDAE 33, 77, 123, 794-799 Anostomidae 21, 33, 50, 131, 168, 184-201, 202 Achirus 4, 33, 794, 796, 797 Anostomus 131, 184, 185, 188-191 Achirus achirus 4, 33, 794, 796, 797 Anostomus anostomus 21, 185, 188, 189 Achirus declivis 33, 794, 796 Anostomus brevior 21, 185, 188, 189 Achirus lineatus 796 Anostomus ternetzi 21, 117, 185, 188-191 Acipenser 5 Aphyocharacidium melandetum 22, 232, 236, 237 Acnodon 23, 48, 288-292 APHYOCHARACINAE 23, 132, 304, 305 Acnodon oligacanthus 23, 48, 289-292 Aphyocharax erythrurus 23, 132, 304, 305 ACTINOPTERYGII 8 Apionichthys dumerili 33, 794, 796-798 Adontosternarchus 602 Apistogramma 720, 723, 728-731, 756 Aequidens 31, 724, 726-729, 750, 752 Apistogramma ortmanni 31, 723, 728-730 Aequidens geayi 750 Apistogramma steindachneri 31, 41, 69, 79, 723, Aequidens paloemeuensis 31, 724, 726, 727 730, 731 Aequidens potaroensis 726 apteronotidae 29, 124, 602-607 Aequidens tetramerus 31, 724, 728, 729 Apteronotus albifrons 4, 29, -

Information Sheet on Ramsar Wetlands (RIS) – 2009-2012 Version Available for Download From
Information Sheet on Ramsar Wetlands (RIS) – 2009-2012 version Available for download from http://www.ramsar.org/ris/key_ris_index.htm. Categories approved by Recommendation 4.7 (1990), as amended by Resolution VIII.13 of the 8th Conference of the Contracting Parties (2002) and Resolutions IX.1 Annex B, IX.6, IX.21 and IX. 22 of the 9th Conference of the Contracting Parties (2005). Notes for compilers: 1. The RIS should be completed in accordance with the attached Explanatory Notes and Guidelines for completing the Information Sheet on Ramsar Wetlands. Compilers are strongly advised to read this guidance before filling in the RIS. 2. Further information and guidance in support of Ramsar site designations are provided in the Strategic Framework and guidelines for the future development of the List of Wetlands of International Importance (Ramsar Wise Use Handbook 14, 3rd edition). A 4th edition of the Handbook is in preparation and will be available in 2009. 3. Once completed, the RIS (and accompanying map(s)) should be submitted to the Ramsar Secretariat. Compilers should provide an electronic (MS Word) copy of the RIS and, where possible, digital copies of all maps. 1. Name and address of the compiler of this form: FOR OFFICE USE ONLY. DD MM YY Beatriz de Aquino Ribeiro - Bióloga - Analista Ambiental / [email protected], (95) Designation date Site Reference Number 99136-0940. Antonio Lisboa - Geógrafo - MSc. Biogeografia - Analista Ambiental / [email protected], (95) 99137-1192. Instituto Chico Mendes de Conservação da Biodiversidade - ICMBio Rua Alfredo Cruz, 283, Centro, Boa Vista -RR. CEP: 69.301-140 2. -

Effects of Deforestation on Headwater Stream Fish Assemblages in the Upper Xingu River Basin, Southeastern Amazonia
Neotropical Ichthyology, 17(1): e180099, 2019 Journal homepage: www.scielo.br/ni DOI: 10.1590/1982-0224-20180099 Published online: 31 January 2019 (ISSN 1982-0224) Copyright © 2019 Sociedade Brasileira de Ictiologia Printed: 30 March 2019 (ISSN 1679-6225) Original article Effects of deforestation on headwater stream fish assemblages in the Upper Xingu River Basin, Southeastern Amazonia Paulo Ilha1,2, Sergio Rosso1 and Luis Schiesari3 The expansion of the Amazonian agricultural frontier represents the most extensive land cover change in the world, detrimentally affecting stream ecosystems which collectively harbor the greatest diversity of freshwater fish on the planet. Our goal was to test the hypotheses that deforestation affects the abundance, richness, and taxonomic structure of headwater stream fish assemblages in the Upper Xingu River Basin, in Southeastern Amazonia. Standardized sampling surveys in replicated first order streams demonstrated that deforestation strongly influences fish assemblage structure. Deforested stream reaches had twice the fish abundance than reference stream reaches in primary forests. These differences in assemblage structure were largely driven by increases in the abundance of a handful of species, as no influence of deforestation on species richness was observed. Stream canopy cover was the strongest predictor of assemblage structure, possibly by a combination of direct and indirect effects on the provision of forest detritus, food resources, channel morphology, and micro-climate regulation. Given the dynamic nature of change in land cover and use in the region, this article is an important contribution to the understanding of the effects of deforestation on Amazonian stream fish, and their conservation. Keywords: Arc of Deforestation, Canopy, Land use, Ichthyofauna, Vegetation cover. -

Global Catfish Biodiversity 17
American Fisheries Society Symposium 77:15–37, 2011 © 2011 by the American Fisheries Society Global Catfi sh Biodiversity JONATHAN W. ARMBRUSTER* Department of Biological Sciences, Auburn University 331 Funchess, Auburn University, Alabama 36849, USA Abstract.—Catfi shes are a broadly distributed order of freshwater fi shes with 3,407 cur- rently valid species. In this paper, I review the different clades of catfi shes, all catfi sh fami- lies, and provide information on some of the more interesting aspects of catfi sh biology that express the great diversity that is present in the order. I also discuss the results of the widely successful All Catfi sh Species Inventory Project. Introduction proximately 10.8% of all fi shes and 5.5% of all ver- tebrates are catfi shes. Renowned herpetologist and ecologist Archie Carr’s But would every one be able to identify the 1941 parody of dichotomous keys, A Subjective Key loricariid catfi sh Pseudancistrus pectegenitor as a to the Fishes of Alachua County, Florida, begins catfi sh (Figure 2A)? It does not have scales, but it with “Any damn fool knows a catfi sh.” Carr is right does have bony plates. It is very fl at, and its mouth but only in part. Catfi shes (the Siluriformes) occur has long jaws but could not be called large. There is on every continent (even fossils are known from a barbel, but you might not recognize it as one as it Antarctica; Figure 1); and the order is extremely is just a small extension of the lip. There are spines well supported by numerous complex synapomor- at the front of the dorsal and pectoral fi ns, but they phies (shared, derived characteristics; Fink and are not sharp like in the typical catfi sh. -

State of the Amazon: Freshwater Connectivity and Ecosystem Health WWF LIVING AMAZON INITIATIVE SUGGESTED CITATION
REPORT LIVING AMAZON 2015 State of the Amazon: Freshwater Connectivity and Ecosystem Health WWF LIVING AMAZON INITIATIVE SUGGESTED CITATION Macedo, M. and L. Castello. 2015. State of the Amazon: Freshwater Connectivity and Ecosystem Health; edited by D. Oliveira, C. C. Maretti and S. Charity. Brasília, Brazil: WWF Living Amazon Initiative. 136pp. PUBLICATION INFORMATION State of the Amazon Series editors: Cláudio C. Maretti, Denise Oliveira and Sandra Charity. This publication State of the Amazon: Freshwater Connectivity and Ecosystem Health: Publication editors: Denise Oliveira, Cláudio C. Maretti, and Sandra Charity. Publication text editors: Sandra Charity and Denise Oliveira. Core Scientific Report (chapters 1-6): Written by Marcia Macedo and Leandro Castello; scientific assessment commissioned by WWF Living Amazon Initiative (LAI). State of the Amazon: Conclusions and Recommendations (chapter 7): Cláudio C. Maretti, Marcia Macedo, Leandro Castello, Sandra Charity, Denise Oliveira, André S. Dias, Tarsicio Granizo, Karen Lawrence WWF Living Amazon Integrated Approaches for a More Sustainable Development in the Pan-Amazon Freshwater Connectivity Cláudio C. Maretti; Sandra Charity; Denise Oliveira; Tarsicio Granizo; André S. Dias; and Karen Lawrence. Maps: Paul Lefebvre/Woods Hole Research Center (WHRC); Valderli Piontekwoski/Amazon Environmental Research Institute (IPAM, Portuguese acronym); and Landscape Ecology Lab /WWF Brazil. Photos: Adriano Gambarini; André Bärtschi; Brent Stirton/Getty Images; Denise Oliveira; Edison Caetano; and Ecosystem Health Fernando Pelicice; Gleilson Miranda/Funai; Juvenal Pereira; Kevin Schafer/naturepl.com; María del Pilar Ramírez; Mark Sabaj Perez; Michel Roggo; Omar Rocha; Paulo Brando; Roger Leguen; Zig Koch. Front cover Mouth of the Teles Pires and Juruena rivers forming the Tapajós River, on the borders of Mato Grosso, Amazonas and Pará states, Brazil. -
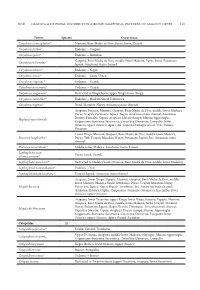
DAGOSTA & DE PINNA: DISTRIBUTION & BIOGEOGRAPHICAL PATTERNS of AMAZON FISHES Taxon Species Occurrence Corydoras Stenocep
2019 DAGOSTA & DE PINNA: DISTRIBUTION & BIOGEOGRAPHICAL PATTERNS OF AMAZON FISHES 113 Taxon Species Occurrence Corydoras stenocephalus* Mamoré, Beni-Madre de Dios, Purus, Juruá, Ucayali Corydoras sterbai* Endemic – Guaporé Corydoras sychri* Endemic – Marañon Guaporé, Beni-Madre de Dios, middle-lower Madeira, Purus, Juruá, Putumayo, Corydoras trilineatus* Japurá, Amazonas main channel Corydoras tukano* Endemic – Negro Corydoras urucu* Endemic – Coari-Urucu Corydoras virginiae* Endemic – Ucayali Corydoras weitzmani* Endemic – Ucayali Corydoras xinguensis* Restricted to Xingu basin (upper Xingu, lower Xingu) Corydoras zawadzkii* Endemic – Madeira Shield Tributaries Corydoras zygatus* Juruá, Marañon-Nanay, Amazonas main channel Araguaia, Juruena, Mamoré, Guaporé, Beni-Madre de Dios, middle-lower Madeira, Purus, Ucayali, Putumayo, Japurá, Negro, Amazonas main channel, Amazonas Estuary, Parnaíba, Capim, Araguari-Macari-Amapá, Maroni-Approuague, Hoplosternum littorale Coppename-Suriname-Saramacca, Corentyne-Demerara, Essequibo, lower Orinoco, upper Orinoco, Apure, Atl. Coastal Drainages of Col. Ven., Paraná- Paraguay Lower Xingu, Mamoré, Guaporé, Beni-Madre de Dios, middle-lower Madeira, Dianema longibarbis* Purus, Tefé, Ucayali, Marañon-Nanay, Putumayo, Japurá, Jari, Amazonas main channel Dianema urostriatum* Middle-lower Madeira, Amazonas main channel Lepthoplosternum Purus, Juruá, Ucayali altamazonicum* Lepthoplosternum beni* Restricted to Madeira basin (Mamoré, Beni-Madre de Dios, middle-lower Madeira) Lepthoplosternum stellatum* Endemic -

Making a Big Splash with Louisiana Fishes
Making a Big Splash with Louisiana Fishes Written and Designed by Prosanta Chakrabarty, Ph.D., Sophie Warny, Ph.D., and Valerie Derouen LSU Museum of Natural Science To those young people still discovering their love of nature... Note to parents, teachers, instructors, activity coordinators and to all the fishermen in us: This book is a companion piece to Making a Big Splash with Louisiana Fishes, an exhibit at Louisiana State Universi- ty’s Museum of Natural Science (MNS). Located in Foster Hall on the main campus of LSU, this exhibit created in 2012 contains many of the elements discussed in this book. The MNS exhibit hall is open weekdays, from 8 am to 4 pm, when the LSU campus is open. The MNS visits are free of charge, but call our main office at 225-578-2855 to schedule a visit if your group includes 10 or more students. Of course the book can also be enjoyed on its own and we hope that you will enjoy it on your own or with your children or students. The book and exhibit was funded by the Louisiana Board Of Regents, Traditional Enhancement Grant - Education: Mak- ing a Big Splash with Louisiana Fishes: A Three-tiered Education Program and Museum Exhibit. Funding was obtained by LSUMNS Curators’ Sophie Warny and Prosanta Chakrabarty who designed the exhibit with Southwest Museum Services who built it in 2012. The oarfish in the exhibit was created by Carolyn Thome of the Smithsonian, and images exhibited here are from Curator Chakrabarty unless noted elsewhere (see Appendix II). -

Redalyc.Survey of Fish Species from Plateau Streams of the Miranda
Biota Neotropica ISSN: 1676-0611 [email protected] Instituto Virtual da Biodiversidade Brasil Silva Ferreira, Fabiane; Serra do Vale Duarte, Gabriela; Severo-Neto, Francisco; Froehlich, Otávio; Rondon Súarez, Yzel Survey of fish species from plateau streams of the Miranda River Basin in the Upper Paraguay River Region, Brazil Biota Neotropica, vol. 17, núm. 3, 2017, pp. 1-9 Instituto Virtual da Biodiversidade Campinas, Brasil Available in: http://www.redalyc.org/articulo.oa?id=199152588008 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Biota Neotropica 17(3): e20170344, 2017 ISSN 1676-0611 (online edition) Inventory Survey of fish species from plateau streams of the Miranda River Basin in the Upper Paraguay River Region, Brazil Fabiane Silva Ferreira1, Gabriela Serra do Vale Duarte1, Francisco Severo-Neto2, Otávio Froehlich3 & Yzel Rondon Súarez4* 1Universidade Estadual de Mato Grosso do Sul, Centro de Estudos em Recursos Naturais, Dourados, MS, Brazil 2Universidade Federal de Mato Grosso do Sul, Laboratório de Zoologia, Campo Grande, CG, Brazil 3Universidade Federal de Mato Grosso do Sul, Departamento de Zoologia, Campo Grande, CG, Brazil 4Universidade Estadual de Mato Grosso do Sul, Centro de Estudos em Recursos Naturais, Lab. Ecologia, Dourados, MS, Brazil *Corresponding author: Yzel Rondon Súarez, e-mail: [email protected] FERREIRA, F. S., DUARTE, G. S. V., SEVERO-NETO, F., FROEHLICH O., SÚAREZ, Y. R. Survey of fish species from plateau streams of the Miranda River Basin in the Upper Paraguay River Region, Brazil.