Ecography ECOG-02845 Leitão, R
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Information Sheet on Ramsar Wetlands (RIS) – 2009-2012 Version Available for Download From
Information Sheet on Ramsar Wetlands (RIS) – 2009-2012 version Available for download from http://www.ramsar.org/ris/key_ris_index.htm. Categories approved by Recommendation 4.7 (1990), as amended by Resolution VIII.13 of the 8th Conference of the Contracting Parties (2002) and Resolutions IX.1 Annex B, IX.6, IX.21 and IX. 22 of the 9th Conference of the Contracting Parties (2005). Notes for compilers: 1. The RIS should be completed in accordance with the attached Explanatory Notes and Guidelines for completing the Information Sheet on Ramsar Wetlands. Compilers are strongly advised to read this guidance before filling in the RIS. 2. Further information and guidance in support of Ramsar site designations are provided in the Strategic Framework and guidelines for the future development of the List of Wetlands of International Importance (Ramsar Wise Use Handbook 14, 3rd edition). A 4th edition of the Handbook is in preparation and will be available in 2009. 3. Once completed, the RIS (and accompanying map(s)) should be submitted to the Ramsar Secretariat. Compilers should provide an electronic (MS Word) copy of the RIS and, where possible, digital copies of all maps. 1. Name and address of the compiler of this form: FOR OFFICE USE ONLY. DD MM YY Beatriz de Aquino Ribeiro - Bióloga - Analista Ambiental / [email protected], (95) Designation date Site Reference Number 99136-0940. Antonio Lisboa - Geógrafo - MSc. Biogeografia - Analista Ambiental / [email protected], (95) 99137-1192. Instituto Chico Mendes de Conservação da Biodiversidade - ICMBio Rua Alfredo Cruz, 283, Centro, Boa Vista -RR. CEP: 69.301-140 2. -

Effects of Deforestation on Headwater Stream Fish Assemblages in the Upper Xingu River Basin, Southeastern Amazonia
Neotropical Ichthyology, 17(1): e180099, 2019 Journal homepage: www.scielo.br/ni DOI: 10.1590/1982-0224-20180099 Published online: 31 January 2019 (ISSN 1982-0224) Copyright © 2019 Sociedade Brasileira de Ictiologia Printed: 30 March 2019 (ISSN 1679-6225) Original article Effects of deforestation on headwater stream fish assemblages in the Upper Xingu River Basin, Southeastern Amazonia Paulo Ilha1,2, Sergio Rosso1 and Luis Schiesari3 The expansion of the Amazonian agricultural frontier represents the most extensive land cover change in the world, detrimentally affecting stream ecosystems which collectively harbor the greatest diversity of freshwater fish on the planet. Our goal was to test the hypotheses that deforestation affects the abundance, richness, and taxonomic structure of headwater stream fish assemblages in the Upper Xingu River Basin, in Southeastern Amazonia. Standardized sampling surveys in replicated first order streams demonstrated that deforestation strongly influences fish assemblage structure. Deforested stream reaches had twice the fish abundance than reference stream reaches in primary forests. These differences in assemblage structure were largely driven by increases in the abundance of a handful of species, as no influence of deforestation on species richness was observed. Stream canopy cover was the strongest predictor of assemblage structure, possibly by a combination of direct and indirect effects on the provision of forest detritus, food resources, channel morphology, and micro-climate regulation. Given the dynamic nature of change in land cover and use in the region, this article is an important contribution to the understanding of the effects of deforestation on Amazonian stream fish, and their conservation. Keywords: Arc of Deforestation, Canopy, Land use, Ichthyofauna, Vegetation cover. -

Global Catfish Biodiversity 17
American Fisheries Society Symposium 77:15–37, 2011 © 2011 by the American Fisheries Society Global Catfi sh Biodiversity JONATHAN W. ARMBRUSTER* Department of Biological Sciences, Auburn University 331 Funchess, Auburn University, Alabama 36849, USA Abstract.—Catfi shes are a broadly distributed order of freshwater fi shes with 3,407 cur- rently valid species. In this paper, I review the different clades of catfi shes, all catfi sh fami- lies, and provide information on some of the more interesting aspects of catfi sh biology that express the great diversity that is present in the order. I also discuss the results of the widely successful All Catfi sh Species Inventory Project. Introduction proximately 10.8% of all fi shes and 5.5% of all ver- tebrates are catfi shes. Renowned herpetologist and ecologist Archie Carr’s But would every one be able to identify the 1941 parody of dichotomous keys, A Subjective Key loricariid catfi sh Pseudancistrus pectegenitor as a to the Fishes of Alachua County, Florida, begins catfi sh (Figure 2A)? It does not have scales, but it with “Any damn fool knows a catfi sh.” Carr is right does have bony plates. It is very fl at, and its mouth but only in part. Catfi shes (the Siluriformes) occur has long jaws but could not be called large. There is on every continent (even fossils are known from a barbel, but you might not recognize it as one as it Antarctica; Figure 1); and the order is extremely is just a small extension of the lip. There are spines well supported by numerous complex synapomor- at the front of the dorsal and pectoral fi ns, but they phies (shared, derived characteristics; Fink and are not sharp like in the typical catfi sh. -
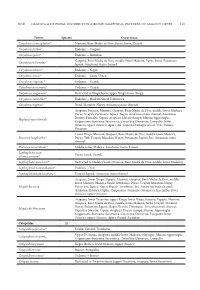
DAGOSTA & DE PINNA: DISTRIBUTION & BIOGEOGRAPHICAL PATTERNS of AMAZON FISHES Taxon Species Occurrence Corydoras Stenocep
2019 DAGOSTA & DE PINNA: DISTRIBUTION & BIOGEOGRAPHICAL PATTERNS OF AMAZON FISHES 113 Taxon Species Occurrence Corydoras stenocephalus* Mamoré, Beni-Madre de Dios, Purus, Juruá, Ucayali Corydoras sterbai* Endemic – Guaporé Corydoras sychri* Endemic – Marañon Guaporé, Beni-Madre de Dios, middle-lower Madeira, Purus, Juruá, Putumayo, Corydoras trilineatus* Japurá, Amazonas main channel Corydoras tukano* Endemic – Negro Corydoras urucu* Endemic – Coari-Urucu Corydoras virginiae* Endemic – Ucayali Corydoras weitzmani* Endemic – Ucayali Corydoras xinguensis* Restricted to Xingu basin (upper Xingu, lower Xingu) Corydoras zawadzkii* Endemic – Madeira Shield Tributaries Corydoras zygatus* Juruá, Marañon-Nanay, Amazonas main channel Araguaia, Juruena, Mamoré, Guaporé, Beni-Madre de Dios, middle-lower Madeira, Purus, Ucayali, Putumayo, Japurá, Negro, Amazonas main channel, Amazonas Estuary, Parnaíba, Capim, Araguari-Macari-Amapá, Maroni-Approuague, Hoplosternum littorale Coppename-Suriname-Saramacca, Corentyne-Demerara, Essequibo, lower Orinoco, upper Orinoco, Apure, Atl. Coastal Drainages of Col. Ven., Paraná- Paraguay Lower Xingu, Mamoré, Guaporé, Beni-Madre de Dios, middle-lower Madeira, Dianema longibarbis* Purus, Tefé, Ucayali, Marañon-Nanay, Putumayo, Japurá, Jari, Amazonas main channel Dianema urostriatum* Middle-lower Madeira, Amazonas main channel Lepthoplosternum Purus, Juruá, Ucayali altamazonicum* Lepthoplosternum beni* Restricted to Madeira basin (Mamoré, Beni-Madre de Dios, middle-lower Madeira) Lepthoplosternum stellatum* Endemic -

Redalyc.Survey of Fish Species from Plateau Streams of the Miranda
Biota Neotropica ISSN: 1676-0611 [email protected] Instituto Virtual da Biodiversidade Brasil Silva Ferreira, Fabiane; Serra do Vale Duarte, Gabriela; Severo-Neto, Francisco; Froehlich, Otávio; Rondon Súarez, Yzel Survey of fish species from plateau streams of the Miranda River Basin in the Upper Paraguay River Region, Brazil Biota Neotropica, vol. 17, núm. 3, 2017, pp. 1-9 Instituto Virtual da Biodiversidade Campinas, Brasil Available in: http://www.redalyc.org/articulo.oa?id=199152588008 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Biota Neotropica 17(3): e20170344, 2017 ISSN 1676-0611 (online edition) Inventory Survey of fish species from plateau streams of the Miranda River Basin in the Upper Paraguay River Region, Brazil Fabiane Silva Ferreira1, Gabriela Serra do Vale Duarte1, Francisco Severo-Neto2, Otávio Froehlich3 & Yzel Rondon Súarez4* 1Universidade Estadual de Mato Grosso do Sul, Centro de Estudos em Recursos Naturais, Dourados, MS, Brazil 2Universidade Federal de Mato Grosso do Sul, Laboratório de Zoologia, Campo Grande, CG, Brazil 3Universidade Federal de Mato Grosso do Sul, Departamento de Zoologia, Campo Grande, CG, Brazil 4Universidade Estadual de Mato Grosso do Sul, Centro de Estudos em Recursos Naturais, Lab. Ecologia, Dourados, MS, Brazil *Corresponding author: Yzel Rondon Súarez, e-mail: [email protected] FERREIRA, F. S., DUARTE, G. S. V., SEVERO-NETO, F., FROEHLICH O., SÚAREZ, Y. R. Survey of fish species from plateau streams of the Miranda River Basin in the Upper Paraguay River Region, Brazil. -

Redalyc.Checklist of the Freshwater Fishes of Colombia
Biota Colombiana ISSN: 0124-5376 [email protected] Instituto de Investigación de Recursos Biológicos "Alexander von Humboldt" Colombia Maldonado-Ocampo, Javier A.; Vari, Richard P.; Saulo Usma, José Checklist of the Freshwater Fishes of Colombia Biota Colombiana, vol. 9, núm. 2, 2008, pp. 143-237 Instituto de Investigación de Recursos Biológicos "Alexander von Humboldt" Bogotá, Colombia Available in: http://www.redalyc.org/articulo.oa?id=49120960001 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Biota Colombiana 9 (2) 143 - 237, 2008 Checklist of the Freshwater Fishes of Colombia Javier A. Maldonado-Ocampo1; Richard P. Vari2; José Saulo Usma3 1 Investigador Asociado, curador encargado colección de peces de agua dulce, Instituto de Investigación de Recursos Biológicos Alexander von Humboldt. Claustro de San Agustín, Villa de Leyva, Boyacá, Colombia. Dirección actual: Universidade Federal do Rio de Janeiro, Museu Nacional, Departamento de Vertebrados, Quinta da Boa Vista, 20940- 040 Rio de Janeiro, RJ, Brasil. [email protected] 2 Division of Fishes, Department of Vertebrate Zoology, MRC--159, National Museum of Natural History, PO Box 37012, Smithsonian Institution, Washington, D.C. 20013—7012. [email protected] 3 Coordinador Programa Ecosistemas de Agua Dulce WWF Colombia. Calle 61 No 3 A 26, Bogotá D.C., Colombia. [email protected] Abstract Data derived from the literature supplemented by examination of specimens in collections show that 1435 species of native fishes live in the freshwaters of Colombia. -
Checklist of the Ichthyofauna of the Rio Negro Basin in the Brazilian Amazon
A peer-reviewed open-access journal ZooKeys 881: 53–89Checklist (2019) of the ichthyofauna of the Rio Negro basin in the Brazilian Amazon 53 doi: 10.3897/zookeys.881.32055 CHECKLIST http://zookeys.pensoft.net Launched to accelerate biodiversity research Checklist of the ichthyofauna of the Rio Negro basin in the Brazilian Amazon Hélio Beltrão1, Jansen Zuanon2, Efrem Ferreira2 1 Universidade Federal do Amazonas – UFAM; Pós-Graduação em Ciências Pesqueiras nos Trópicos PPG- CIPET; Av. Rodrigo Otávio Jordão Ramos, 6200, Coroado I, Manaus-AM, Brazil 2 Instituto Nacional de Pesquisas da Amazônia – INPA; Coordenação de Biodiversidade; Av. André Araújo, 2936, Caixa Postal 478, CEP 69067-375, Manaus, Amazonas, Brazil Corresponding author: Hélio Beltrão ([email protected]) Academic editor: M. E. Bichuette | Received 30 November 2018 | Accepted 2 September 2019 | Published 17 October 2019 http://zoobank.org/B45BD285-2BD4-45FD-80C1-4B3B23F60AEA Citation: Beltrão H, Zuanon J, Ferreira E (2019) Checklist of the ichthyofauna of the Rio Negro basin in the Brazilian Amazon. ZooKeys 881: 53–89. https://doi.org/10.3897/zookeys.881.32055 Abstract This study presents an extensive review of published and unpublished occurrence records of fish species in the Rio Negro drainage system within the Brazilian territory. The data was gathered from two main sources: 1) litterature compilations of species occurrence records, including original descriptions and re- visionary studies; and 2) specimens verification at the INPA fish collection. The results reveal a rich and diversified ichthyofauna, with 1,165 species distributed in 17 orders (+ two incertae sedis), 56 families, and 389 genera. A large portion of the fish fauna (54.3% of the species) is composed of small-sized fishes < 10 cm in standard length. -

Trichomycterus Punctulatus ERSS
Trichomycterus punctulatus (a catfish, no common name) Ecological Risk Screening Summary U.S. Fish and Wildlife Service, December 2016 Revised, February 2018 Web Version, 2/21/2020 Photo: © Erling Holm, Royal Ontario Museum, used by permission. 1 Native Range and Status in the United States Native Range From Froese and Pauly (2016): “South America: western Peru.” From Eschmeyer et al. (2016): “Distribution: Western Peru.” Status in the United States This species has not been reported as introduced or established in the United States. There is no indication that this species is in trade in the United States. From Arizona Secretary of State (2006): “Fish listed below are restricted live wildlife [in Arizona] as defined in R12-4-401. […] South American parasitic catfish, all species of the family Trichomycteridae and Cetopsidae […]” 1 From Dill and Cordone (1997): “[…] At the present time, 22 families of bony and cartilaginous fishes are listed [as prohibited in California], e.g. all parasitic catfishes (family Trichomycteridae) […]” From FFWCC (2019): “Nonnative Conditional species (formerly referred to as restricted species) and Prohibited species are considered to be dangerous to Florida’s native species and habitats or could pose threats to the health and welfare of the people of Florida. These species are not allowed to be personally possessed, but can be imported and possessed by permit for research or public exhibition; Conditional species may also be possessed by permit for commercial sales. Facilities where Conditional or Prohibited species are held must meet certain biosecurity criteria to prevent escape.” Trichomycterus punctulatus is listed as a Prohibited species in Florida. -

Nevada Aquatic Invasive Species Management Plan 2017
Nevada Aquatic Invasive Species Management Plan 2017 KNOTT CREEK RESERVOIR, NEVADA This Aquatic Invasive Species Management Plan is part of a multi-stakeholder collaboration with the Nevada Department of Wildlife, and Nevada agencies and organizations and with input from the general public. LAKE MEAD, NEVADA This product was prepared by: Suggested citation: Nevada Department of Wildlife. 2017 Nevada Aquatic Invasive Species Management Plan. Reno. Pp 106. State of Nevada BRIAN SANDOVAL, GOVERNOR Signature Date State of Nevada TONY WASLEY, DIRECTOR, NEVADA DEPARTMENT OF WILDLIFE Signature Date State of Nevada GRANT WALLACE, CHAIRMAN, BOARD OF WILDLIFE COMMISSIONERS, NEVADA DEPARTMENT OF WILDLIFE Signature Date TABLE OF CONTENTS LIST OF FIGURES 6 LIST OF TABLES 6 ACRONYMS 7 EXECUTIVE SUMMARY 8 INTRODUCTION 10 PLAN PURPOSE 10 PLAN DEVELOPMENT 11 PRIORITIZING MANAGEMENT 12 GEOGRAPHIC SCOPE 13 REGIONAL GEOGRAPHIC DYNAMICS 13 CLIMATE CHANGE AND INVASIVE SPECIES 19 RAPID RESPONSE STRATEGY 23 EXISTING AUTHORITIES AND PROGRAMS 24 STATE 26 TRIBAL 29 FEDERAL 29 REGIONAL 33 GAPS AND CHALLENGES 34 AIS MANAGEMENT APPROACH 35 PROBLEM DEFINITION 36 RECENT AIS PROBLEMS 36 AIS OF CONCERN AND TYPES 36 AIS OF FOCUS IN NEVADA 37 ANIMALS 37 AQUATIC AND RIPARIAN PLANTS 37 PATHOGENS 37 PATHWAYS OF INTRODUCTION 38 MANAGEMENT PLAN GOALS AND OBJECTIVES 40 TABLE OF CONTENTS OBJECTIVE A: OVERSIGHT AND COORDINATION 40 OBJECTIVE B: PREVENTION 40 OBJECTIVE C: OUTREACH AND EDUCATION 40 OBJECTIVE D: MONITORING, EARLY DETECTION, AND 40 RESPONSE OBJECTIVE E: LONG-TERM CONTROL -

Sound Production in Two Loricariid Catfishes Amanda Lynn Webb Western Kentucky University, [email protected]
Western Kentucky University TopSCHOLAR® Masters Theses & Specialist Projects Graduate School 8-2011 Sound Production in Two Loricariid Catfishes Amanda Lynn Webb Western Kentucky University, [email protected] Follow this and additional works at: http://digitalcommons.wku.edu/theses Part of the Biology Commons, and the Structural Biology Commons Recommended Citation Webb, Amanda Lynn, "Sound Production in Two Loricariid Catfishes" (2011). Masters Theses & Specialist Projects. Paper 1089. http://digitalcommons.wku.edu/theses/1089 This Thesis is brought to you for free and open access by TopSCHOLAR®. It has been accepted for inclusion in Masters Theses & Specialist Projects by an authorized administrator of TopSCHOLAR®. For more information, please contact [email protected]. SOUND PRODUCTION IN TWO LORICARIID CATFISHES A Thesis Presented to The Faculty of the Department of Biology Western Kentucky University Bowling Green, Kentucky In Partial Fulfillment Of the Requirements for the Degree Master of Science By Amanda Lynn Webb August 2011 ACKNOWLEDGEMENTS First, I would like to thank my parents. Without their love, support, and encouragement, I would not be where I am today. I would also like to thank Dr. Michael Smith for the many wonderful years as my undergraduate and graduate research advisor. I truly appreciate his support and patience and the wonderful opportunities I have had working in his lab. I am also thankful for the support from my committee members, Dr. Sigrid Jacobshagen and Phil Lienesch, along with all the other Biology department faculty and staff. I am grateful to the Western Kentucky University Honors College for their support via an Honors Development Grant. -

Neuroanatomia E Filogenia Da Família Cetopsidae (Osteichthyes, Ostariophysi, Siluriformes) Com Análises Simultâneas De Dados Morfológicos E Moleculares
MUSEU DE ZOOLOGIA DA UNIVERSIDADE DE SÃO PAULO VITOR PIMENTA ABRAHÃO NEUROANATOMIA E FILOGENIA DA FAMÍLIA CETOPSIDAE (OSTEICHTHYES, OSTARIOPHYSI, SILURIFORMES) COM ANÁLISES SIMULTÂNEAS DE DADOS MORFOLÓGICOS E MOLECULARES SÃO PAULO 2018 VITOR PIMENTA ABRAHÃO NEUROANATOMIA E FILOGENIA DA FAMÍLIA CETOPSIDAE (OSTEICHTHYES, OSTARIOPHYSI, SILURIFORMES) COM ANÁLISES SIMULTÂNEAS DE DADOS MORFOLÓGICOS E MOLECULARES TESE DE DOUTORADO MUSEU DE ZOOLOGIA DA UNIVERSIDADE DE SÃO PAULO TESE DE DOUTORADO APRESENTADA COMO UM DOS REQUISITOS PARA A OBTENÇÃO DO TÍTULO DE DOUTOR PELO MUSEU DE ZOOLOGIA DA UNIVERSIDADE DE SÃO PAULO. ÁREA DE CONCENTRAÇÃO: ZOOLOGIA ORIENTADOR: MÁRIO CÉSAR CARDOSO DE PINNA SÃO PAULO 2018 ii AUTORIZO A REPRODUÇÃO E DIVULGAÇÃO TOTAL OU PARCIAL DESTE TRABALHO, POR QUALQUER MEIO CONVENCIONAL OU ELETRÔNICO, PARA FINS DE ESTUDO E PESQUISA, DESDE QUE CITADA A FONTE. I AUTHORIZE THE REPRODUCTION AND DISSEMINATION OF THIS WORK IN PART OR ENTIRELY BY ANY MEANS ELETRONIC OR CONVENTIONAL, FOR STUDY AND RESEARCH, PROVIDE THE SOURCE IS CITED. ii Abrahão, Vitor Pimenta Neuroanatonia e filogenia da família Cetopsidae (Ostariophysis, Siluriformes) com análises simultâneas de dados morfológicos. / Vitor Pimenta Abrahão ; orientador Mário César Cardoso de Pinna. São Paulo, 2018. 367 p. Tese de Doutorado – Programa de Pós-Graduação em Sistemática, Taxonomia e Biodiversidade, Museu de Zoologia, Universidade de São Paulo, 2018. Versão original 1. Neuroanatomia – Cetopsidae 2. Cetopsidae - Filogenia. I. Pinna, Mário César Cardoso de, orient. II. Título. CDU 597.55 CDU 597.551.4 BANCA EXAMINADORA Prof. Dr.__________________________ Instituição:____________________________ Julgamento: _______________________ Assinatura: ___________________________ Prof. Dr.__________________________ Instituição:____________________________ Julgamento: _______________________ Assinatura: ___________________________ Prof. Dr.__________________________ Instituição:____________________________ Julgamento: _______________________ Assinatura: ___________________________ Prof. -

XII (L): L0l - Ll8 Kiel, Juni 1992
AMAZONIANA XII (l): l0l - ll8 Kiel, Juni 1992 Food taboos and folk medicine among fishermen from the Tocantins River (Brazil) by Alpina Begossi- and Francisco Manoel de Souza Braga Dr. Alpina Begossi, Departamento de Ecologia, IB, Universidade Federal do Rio de Janeiro, C.P. 68020, 21941Rio de Janeiro, RI, Brazil. Prof. Dr. Francisco Manoel de Souza Braga, Departamento de Zoologia, Universidade Estadual Paulisø, C.P. 179, 13.500 Rio Claro, SP, Brazil. (Accepted for publicatian: August, 1990). Abstract Fish utilization for food and folk medicine, and fish preference of families from the Tocantins river were studied. Questionnaires werc used in the 234 interviews performed in cities, towns and scatteres houses located along 100 km of river stretch. Curimatí (Prochilodtu nigricans) is the most consumed fish and pacu-manteiga (Mylossorna duriventre) ¡he most preferred species. The fish species avoided are correlated with the species used in folk medicine (r, = 0,54, p < 0.02). Food t¡boos, orfish species not consumed during illness, are also cited. The usefulness of fish species for folk medicine and the piscivorous habits of most fish quoted as not consumed partially explain the food choices of fishermen. These explanations conform to materialist theories in cultural ecology. Keywords: DIeÇ food teboog ñshlng communitleq Brezll, Rlo Toc¡ntins. Introduction Food preferences and avoidances have been a subject of many studies in Anthro- pology and Human Ecology. MESSER (1984) reviewed some of these studies and factors involved in the fmd choice of human populations. The avoidances of some food by people are considered to be based on ideological criteria (SAHLINS 1976) or on materialist reasons (HARRIS L977,1985).