Marine Ecology Progress Series 529:233
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

View/Download
SPARIFORMES · 1 The ETYFish Project © Christopher Scharpf and Kenneth J. Lazara COMMENTS: v. 4.0 - 13 Feb. 2021 Order SPARIFORMES 3 families · 49 genera · 283 species/subspecies Family LETHRINIDAE Emporerfishes and Large-eye Breams 5 genera · 43 species Subfamily Lethrininae Emporerfishes Lethrinus Cuvier 1829 from lethrinia, ancient Greek name for members of the genus Pagellus (Sparidae) which Cuvier applied to this genus Lethrinus amboinensis Bleeker 1854 -ensis, suffix denoting place: Ambon Island, Molucca Islands, Indonesia, type locality (occurs in eastern Indian Ocean and western Pacific from Indonesia east to Marshall Islands and Samoa, north to Japan, south to Western Australia) Lethrinus atkinsoni Seale 1910 patronym not identified but probably in honor of William Sackston Atkinson (1864-ca. 1925), an illustrator who prepared the plates for a paper published by Seale in 1905 and presumably the plates in this 1910 paper as well Lethrinus atlanticus Valenciennes 1830 Atlantic, the only species of the genus (and family) known to occur in the Atlantic Lethrinus borbonicus Valenciennes 1830 -icus, belonging to: Borbon (or Bourbon), early name for Réunion island, western Mascarenes, type locality (occurs in Red Sea and western Indian Ocean from Persian Gulf and East Africa to Socotra, Seychelles, Madagascar, Réunion, and the Mascarenes) Lethrinus conchyliatus (Smith 1959) clothed in purple, etymology not explained, probably referring to “bright mauve” area at central basal part of pectoral fins on living specimens Lethrinus crocineus -

Developing a System of Sustainable Minimum Size Limits for Fiji
Developing a system of sustainable minimum size limits for Fiji Jeremy D. Prince1, Adrian Hordyk2, Sangeeta Mangubhai3, Watisoni Lalavanua3, Ilaitia Tamata4, Jone Tamanitoakula3, Tevita Vodivodi5, Iva Meo5, Diana Divalotu5, Tamari Iobi5, Epeli Loganimoce3, Kalisi Logatabua5, Kalisiana Marama5, Diana Nalasi5, Waisea Naisilisili3, Unaisi Nalasi5, Mosese Naleba3, Pitila Waqainabete5 Introduction with this simplification, setting MSLs in many of the PICTs that are scattered across the expanse of the Pacific Ocean is a The depletion of reef fish stocks across the South Pacific challenging prospect. This attempt has been made difficult poses a major threat to both food security and preservation due to the large numbers of species from catches that have of biodiversity (Newton et al. 2007; Sale and Hixon 2015). not been studied in depth, along with the impracticality of Highly prized large-bodied grouper, snapper, parrotfish and implementing large numbers of species-specific MSLs for an wrasse have become harder to catch and more expensive in assemblage of species that appear to be similar. markets everywhere. Once common, some species are now rare or locally extinct in many places. Researchers are pre- This article describes a study undertaken in Fiji in order dicting that many species face global extinction if effective to address this challenge. The study, which was supported management is not implemented (Sadovy et al. 2003, 2013; primarily by the David and Lucile Packard Foundation and Dulvy and Polunin 2004). Most Pacific Island countries -

Power Supply Procurement Plan
POWER SUPPLY PROCUREMENT PLAN ZAMBOANGA DEL NORTE ELECTRIC COOPERATIVE, INC. POWER SUPPLY PROCUREMENT PLAN In compliance with the Department of Energy’s (DOE) Department Circular No. DC 2018-02-0003, “Adopting and Prescribing the Policy for the Competitive Selection Process in the Procurement by the Distribution Utilities of Power Supply Agreement for the Captive Market” or the Competitive Selection process (CSP) Policy, the Power Supply Procurement Plan (PSPP) Report is hereby created, pursuant to the Section 4 of the said Circular. The PSPP refers to the DUs’ plan for the acquisition of a variety of demand-side and supply-side resources to cost-effectively meet the electricity needs of its customers. The PSPP is an integral part of the Distribution Utilities’ Distribution Development Plan (DDP) and must be submitted to the Department of Energy with supported Board Resolution and/or notarized Secretary’s Certificate. The Third-Party Bids and Awards Committee (TPBAC), Joint TPBAC or Third Party Auctioneer (TPA) shall submit to the DOE and in the case of Electric Cooperatives (ECs), through the National Electrification Administration (NEA) the following: a. Power Supply Procurement Plan; b. Distribution Impact Study/ Load Flow Analysis conducted that served as the basis of the Terms of Reference; and c. Due diligence report of the existing generation plant All Distribution Utilities’ shall follow and submit the attached report to the Department of Energy for posting on the DOE CSP Portal. For ECs such reports shall be submitted to DOE and NEA. The NEA shall review the submitted report within ten (10) working days upon receipt prior to its submission to DOE for posting at the DOE CSP Portal. -

The Regional Development Report Scorecard Xix Joint RDC IX and RPOC IX Resolution Xxi Foreword Xxiii Message Xxiv Executive Summary Xxv
Zamboanga Peninsula 2019Regional Development Report Table of Contents Acronyms and Abbreviations iii List of Tables and Figures xi The Regional Development Report Scorecard xix Joint RDC IX and RPOC IX Resolution xxi Foreword xxiii Message xxiv Executive Summary xxv Chapter 02 Global and Regional Trends and Prospects 1 Chapter 03 Overlay of Economic Growth, Demographic Trends and 5 Physical Characteristics Chapter 04 Zamboanga Peninsula Regional Development Plan 2017-2022 9 Overall Framework Chapter 05 Ensuring People-Centered, Clean and Efficient Governance 13 Chapter 06 Pursuing Swift and Fair Administration of Justice 21 Chapter 07 Promoting Philippine Culture and Values 29 Chapter 08 Expanding Economic Opportunities in Agriculture, Forestry, 33 and Fisheries Chapter 09 Expanding Economic Opportunities in Industry and Services 49 through Trabaho at Negosyo Chapter 10 Accelerating Human Capital Development 57 Chapter 11 Reducing Vulnerability of Individuals and Families 67 Chapter 12 Building Safe and Secure Communities 71 Chapter 13 Reaching for the Demographic Dividend 75 Chapter 14 Vigorously Advancing Science, Technology and Innovation 79 Chapter 15 Ensuring Sound Macroeconomic Policy 85 Chapter 17 Attaining Just and Lasting Peace 95 Chapter 18 Ensuring Security, Public Order and Safety 105 Chapter 19 Accelerating Infrastructure Development 117 Chapter 20 Ensuring Ecological Integrity, Clean and Healthy 133 Environment Chapter 22 Plan Implementation and Monitoring 145 Glossary of Terms 153 2019 Zamboanga Peninsula Regional Development -

Djrmh-May 2018
Republic of the Philippines DR. JOSE RIZAL MEMORIAL HOSPITAL - Lawaan, Dapitan City MEDICAL ASSISTANCE PROGRAM FUND UTILIZATION REPORT As of May 2018 Name of Hospital: Dr. Jose Rizal Memorial Hospital Month: May Date Name of Patient Address Age Type of Assistance Amount Provided 1 05/01/2018 JENREY CADORNA Talisay, Dapitan City 12 X-ray 350.00 2 05/01/2018 MELCHOR MONTE Sulangon, Dapitan City 73 Laboratory/Medicines 1,450.10 3 05/01/2018 KINGBERT CARDENAS Rizal, Z.N 15 Hospital Bill 2,447.10 4 05/01/2018 GEERA CALAGUAN Dapitan City 16 Hospital Bill 4,614.45 5 05/01/2018 KINGBERT CARDENAS North Mapang, Rizal, Z.N 15 Medicines 226.80 6 05/01/2018 OSCAR GAHISAN Banonong, Dapitan City 74 Hospital Bill 14,955.44 7 05/01/2018 SATURNINA COLATA Liyang, Dapitan City 66 Hospital Bill 18,869.87 8 05/01/2018 NIMFLOR BAIT-IT Villahermosa, Polanco, Z.N 46 X-ray/Laboratory 2,200.50 9 05/01/2018 CONRADA BAIT-IT Burgos, Dapitan City 75 Hospital Bill 24,440.04 10 05/01/2018 RODRIGO FANO Talisay, Dapitan City 74 Hospital Bill 21,190.64 11 05/02/2018 MERLYN BAJAMUNDE Adante, Piñan, Z.N 41 Hospital Bill 3,139.90 12 05/02/2018 TITO BAHIAN Napo, Dapitan City 70 Medicines 2,908.50 13 05/02/2018 MICHEL CINCO Napo, Dapitan City 29 Medicines 1,428.50 14 05/02/2018 SATURNINA COLATA Liyang, Dapitan City 66 Medicines 1,413.00 15 05/02/2018 DIOSCORO NAPIAL Piñan, Z.N 74 Medicines/X-ray/Laboratory 2,764.00 16 05/02/2018 TITA CABILIN Bagting, Dapitan City 83 Laboratory 1,190.50 17 05/02/2018 EPEGENIA BULAY-OG Banbanan, Dapitan City 61 Meds.Supplies 240.00 18 05/02/2018 JASMEN SUMALPONG Polo, Dapitan City 9 UTZ 750.00 19 05/02/2018 JOSHUA EMPEYNADO Linabo, Dapitan City 12 Medicines 196.50 20 05/02/2018 ERNESTO GANOT JR. -

Current Status and Prospects of Protected Areas in the Light of the Philippine Biodiversity Conservation Priorities
Proceedings of IUCN/WCPA-EA-4 Taipei Conference March 18-23, 2002, Taipei, Taiwan CURRENT STATUS AND PROSPECTS OF PROTECTED AREAS IN THE LIGHT OF THE PHILIPPINE BIODIVERSITY CONSERVATION PRIORITIES Perry S. Ong, Ph. D. Fellow, Center for Applied Biodiversity Science, CI Science Director, Conservation International Philippines Associate Professor, Institute of Biology, UP Diliman I. INTRODUCTION The Philippines, the world’s second largest archipelago after Indonesia, covers a land area of about 300,000 km2 [1]. It is one of the 17 megadiversity countries, which between themselves contain 70 to 80 percent of global biodiversity [2]. Philippine rainforest is home to more than 1130 terrestrial wildlife species (Table 1) and between 10,000-13,000 species of plants [3] so far recorded, of which more than half are found nowhere else in the world. As such, the Philippines has also been described as Galapagos times ten [4]. It is also one of 25 global biodiversity hotspots [5, 6] with more than 97 percent of its original forest cover lost [7, 8]. In fact more original forests were lost in the last 50 years of the 20th century than what was lost in the previous 450 years combined [9]. Yet more new species are still being discovered on these islands than any other areas on earth in recent times [e.g., see 10, 11] Table 1. Diversity, endemism and conservation status of Philippine wildlife [11, 12, 13 14, 15, 16, 17, 18, 19, 20] No. of No. of Endemic % No. of No. of Threatened Species Species endemics Endemic Species Threatened Species Amphibians 101+ 79+ 78% 24 24 Reptiles 258+ 170+ 66% 8 4 Birds 5761 195+1 34% 74 59 Mammals 204+1, 2 111+1 54% 51 41 Total 1139+ 555+1 49% 157 128 95 Legend: + includes new species (38 species of amphibians, 35 species of reptiles; 15 species of mammals); 1 includes rediscovered species 2 25 species of dolphins, whales and dugong The country’s marine waters cover 2.21 M km2 with a coastline of 22,450 km and an estimated 27,000 km2 of coral reefs [21]. -

Elasmobranch Biodiversity, Conservation and Management Proceedings of the International Seminar and Workshop, Sabah, Malaysia, July 1997
The IUCN Species Survival Commission Elasmobranch Biodiversity, Conservation and Management Proceedings of the International Seminar and Workshop, Sabah, Malaysia, July 1997 Edited by Sarah L. Fowler, Tim M. Reed and Frances A. Dipper Occasional Paper of the IUCN Species Survival Commission No. 25 IUCN The World Conservation Union Donors to the SSC Conservation Communications Programme and Elasmobranch Biodiversity, Conservation and Management: Proceedings of the International Seminar and Workshop, Sabah, Malaysia, July 1997 The IUCN/Species Survival Commission is committed to communicate important species conservation information to natural resource managers, decision-makers and others whose actions affect the conservation of biodiversity. The SSC's Action Plans, Occasional Papers, newsletter Species and other publications are supported by a wide variety of generous donors including: The Sultanate of Oman established the Peter Scott IUCN/SSC Action Plan Fund in 1990. The Fund supports Action Plan development and implementation. To date, more than 80 grants have been made from the Fund to SSC Specialist Groups. The SSC is grateful to the Sultanate of Oman for its confidence in and support for species conservation worldwide. The Council of Agriculture (COA), Taiwan has awarded major grants to the SSC's Wildlife Trade Programme and Conservation Communications Programme. This support has enabled SSC to continue its valuable technical advisory service to the Parties to CITES as well as to the larger global conservation community. Among other responsibilities, the COA is in charge of matters concerning the designation and management of nature reserves, conservation of wildlife and their habitats, conservation of natural landscapes, coordination of law enforcement efforts as well as promotion of conservation education, research and international cooperation. -

Training Manual Series No.15/2018
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by CMFRI Digital Repository DBTR-H D Indian Council of Agricultural Research Ministry of Science and Technology Central Marine Fisheries Research Institute Department of Biotechnology CMFRI Training Manual Series No.15/2018 Training Manual In the frame work of the project: DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals 2015-18 Training Manual In the frame work of the project: DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals 2015-18 Training Manual This is a limited edition of the CMFRI Training Manual provided to participants of the “DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals” organized by the Marine Biotechnology Division of Central Marine Fisheries Research Institute (CMFRI), from 2nd February 2015 - 31st March 2018. Principal Investigator Dr. P. Vijayagopal Compiled & Edited by Dr. P. Vijayagopal Dr. Reynold Peter Assisted by Aditya Prabhakar Swetha Dhamodharan P V ISBN 978-93-82263-24-1 CMFRI Training Manual Series No.15/2018 Published by Dr A Gopalakrishnan Director, Central Marine Fisheries Research Institute (ICAR-CMFRI) Central Marine Fisheries Research Institute PB.No:1603, Ernakulam North P.O, Kochi-682018, India. 2 Foreword Central Marine Fisheries Research Institute (CMFRI), Kochi along with CIFE, Mumbai and CIFA, Bhubaneswar within the Indian Council of Agricultural Research (ICAR) and Department of Biotechnology of Government of India organized a series of training programs entitled “DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals”. -

Profile on Environmental and Social Considerations in Philippines
Profile on Environmental and Social Considerations in Philippines ANNEX September 2011 Japan International Cooperation Agency (JICA) CRE CR(5) 11-014 Table of Contents IUCN Red List of the Philippines (2007) Red List of the Philippine Red Data Book,1997 Threatened Species by the National Laws Philippine Fauna and Flora under CITES APPENDIX, 2011 Protected Areas under the NIPAS Act in the Philippines (as of June, 2011) Environmental Standards CDM Projects in the Philippines (as of March 31, 2011) Project Grouping Matrix for Determination of EIA Report Type EIA Coverage & Requirements Screening Checklists Outlines of Required Documents by PEISS IUCN Red List of the Philippines ,2007 IUCN Red List of the Philippines (2007) # Scientific Name Common Name Category Mammals 1 Acerodon jubatus GOLDEN-CAPPED FRUIT BAT EN 2 Acerodon leucotis PALAWAN FRUIT BAT VU 3 Alionycteris paucidentata MINDANAO PYGMY FRUIT BAT VU 4 Anonymomys mindorensis MINDORO CLIMBING RAT VU 5 Apomys sacobianus LONG-NOSED LUZON FOREST MOUSE VU 6 Apomys gracilirostris LARGE MINDORO FOREST MOUSE VU 7 Archboldomys luzonensis MT ISAROG SHREW-MOUSE EN 8 Axis calamianensis CALAMANIAN DEER EN 9 Bubalus mindorensis MINDORO DWARF BUFFALO CR 10 Cervus alfredi PHILLIPINE SPOTTED DEER EN 11 Chrotomys gonzalesi ISAROG STRIPED SHREW-RAT, CR 12 Chrotomys whiteheadi LUZON STRIPED RAT VU 13 Crateromys australis DINAGAT BUSHY-TAILED CLOUD RAT EN 14 Crateromys schadenbergi GIANT BUSHY-TAILED CLOUD RAT VU 15 Crateromys paulus OILIN BUSHY-TAILED CLOUD RAT CR 16 Crateromys heaneyi PANAY BUSHY-TAILED -

NORTH COAST FISH IDENTIFICATION GUIDE Ben M
NORTH COAST FISH IDENTIFICATION GUIDE Ben M. Rome and Stephen J. Newman Department of Fisheries 3rd floor SGIO Atrium 168-170 St George’s Terrace PERTH WA 6000 Telephone (08) 9482 7333 Facsimile (08) 9482 7389 Website: www.fish.wa.gov.au ABN: 55 689 794 771 Published by Department of Fisheries, Perth, Western Australia. Fisheries Occasional Publications No. 80, September 2010. ISSN: 1447 - 2058 ISBN: 1 921258 90 X Information about this guide he intention of the North Coast Fish Identification Guide is to provide a simple, Teasy to use manual to assist commercial, recreational, charter and customary fishers to identify the most commonly caught marine finfish species in the North Coast Bioregion. This guide is not intended to be a comprehensive taxonomic fish ID guide for all species. It is anticipated that this guide will assist fishers in providing a more comprehensive species level description of their catch and hence assist scientists and managers in understanding any variation in the species composition of catches over both spatial and temporal scales. Fish taxonomy is a dynamic and evolving field. Advances in molecular analytical techniques are resolving many of the relationships and inter-relationships among species, genera and families of fishes. In this guide, we have used and adopted the latest taxonomic nomenclature. Any changes to fish taxonomy will be updated and revised in subsequent editions. The North Coast Bioregion extends from the Ashburton River near Onslow to the Northern Territory border. Within this region there is a diverse range of habitats from mangrove creeks, rivers, offshore islands, coral reef systems to continental shelf and slope waters. -
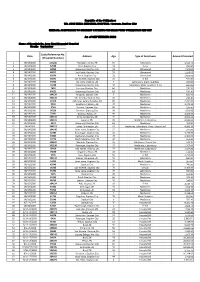
Age Type of Assistance Amount Provided Code/Referrence No
Republic of the Philippines DR. JOSE RIZAL MEMORIAL HOSPITAL - Lawaan, Dapitan City MEDICAL ASSISTANCE TO INDIGENT PATIENTS PROGRAM FUND UTILIZATION REPORT As of SEPTEMBER 2020 Name of Hospital: Dr. Jose Rizal Memorial Hospital Month: September Code/Referrence No. Date Address Age Type of Assistance Amount Provided (Hospital Number) 1 08/14/2020 104106 Totongon, Mutia, ZN 65 Laboratory 2,923.70 2 08/14/2020 32568 Polo, Dapitan City 41 X-ray 350.00 3 08/14/2020 52869 Banonong, Dapitan City 44 Ultrasound 1,500.00 4 08/14/2020 40057 San Pedro, Dapitan City 59 Ultrasound 1,500.00 5 08/14/2020 63596 Potol, Dapitan City 21 Ultrasound 1,500.00 6 08/14/2020 68991 San Vicente, Dapitan City 25 Er Bill 925.00 7 08/14/2020 59893 Sto. Niño, Polanco, ZN 2 Laboratory, Meds. Supplies 265.85 8 08/14/2020 11699 Banonong, Dapitan City 33 Laboratory, Meds. Supplies, X-ray 650.85 9 08/14/2020 7693 Larayan, Dapitan City 60 Medicines 270.20 10 08/14/2020 44021 Banonong, Dapitan City 82 Medicines 715.10 11 08/14/2020 104139 Antipolo, Dapitan City 3 Medicines 635.40 12 08/14/2020 104141 Ma. Cristina, Dapitan City 10 Medicines 212.10 13 08/14/2020 16176 Antonino, Sergio Osmeña, ZN 80 Medicines 2,691.00 14 08/14/2020 9268 Anastacio, Polanco, ZN 20 Medicines 2,172.25 15 08/14/2020 3764 Sinonoc, Dapitan City 81 Medicines 234.00 16 08/14/2020 58226 Sinaman, Dipolog City 38 Medicines 1,950.00 17 08/14/2020 75421 Galukso, Roxas, ZN 40 Medicines 4,660.00 18 08/14/2020 104134 Seres, Katipunan, ZN 25 Medicines 2,562.25 19 08/14/2020 104119 Gutalac, ZN 20 Medicines, Laboratory -

2013 Offshore Banks Assessment Survey
Montara: Barracouta East, Goeree and Vulcan Shoals Survey 2013 Authors: Andrew Heyward, Conrad Speed, Mark Meekan, Mike Cappo, Mark Case, Jamie Colquhoun, Rebecca Fisher, Jessica Meeuwig¹, Ben Radford Report prepared by the Australian Institute of Marine Science for PTTEP Australasia (Ashmore Cartier) Pty. Ltd. in accordance with Contract No. 2013/1153 PERTH AUGUST 2013 ¹ Centre for Marine Futures, University of Western Australia, Perth 1 Australian Institute of Marine Science PMB No 3 PO Box 41775 AIMS (M096) Oceans Institute Townsville MC Qld 4810 Casuarina NT 0811 University of Western Australia Crawley WA 6009 This report should be cited as: Montara: Vulcan, Barracouta East and Goeree Shoals Survey 2013; Heyward et al 2013; Report for PTTEP Australasia (Ashmore Cartier) Pty Ltd. Australian Institute of Marine Science, Perth. (87pp.). © Copyright .Australian Institute of Marine Science (AIMS) and PTTEP Australasia (Ashmore Cartier) Pty Ltd (PTTEPA) 2013 All rights are reserved and no part of this document may be reproduced, stored or copied in any form or by any means whatsoever except with the prior written permission of AIMS or PTTEPA. Acknowledgements Paul Tinkler, Marcus Stowar, Dianne McLean, Skipper and crew of the RV Solander DISCLAIMER While reasonable efforts have been made to ensure that the contents of this document are factually correct, AIMS does not make any representation or give any warranty regarding the accuracy, completeness, currency or suitability for any particular purpose of the information or statements contained in this document. To the extent permitted by law AIMS shall not be liable for any loss, damage, cost or expense that may be occasioned directly or indirectly through the use of or reliance on the contents of this document.