Crohn's Disease of the Foregut
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Clinical Audit on Management of Hematemesis in Children Admitted to Pediatric Gastroenterology and Hepatology Unit of Assiut
Med. J. Cairo Univ., Vol. 86, No. 8, December: 4531-4536, 2018 www.medicaljournalofcairouniversity.net Clinical Audit on Management of Hematemesis in Children Admitted to Pediatric Gastroenterology and Hepatology Unit of Assiut University Children Hospital ESRAA T. AHMED, M.Sc.; FATMA A. ALI, M.D. and NAGLA H. ABU FADDAN, M.D. The Department of Pediatrics, Faculty of Medicine, Assiut University, Assiut, Egypt Abstract Hematemesis: Indicates that the bleeding origin is above the Treitz angle, i.e., that it constitutes an Background: Hematemesis is an uncommon but potentially Upper Gastrointestinal Bleeding (UGIB) [3] . serious and life-threatening clinical condition in children. It indicates that the bleeding origin is above the Treitz angle, The etiology of upper GI bleeding varies by i.e., that it constitutes an Upper Gastrointestinal Bleeding (UGIB). age. The pathophysiology of upper GI bleeding is related to the source of the bleeding. Most clinically Aim of Study: To assess for how much the adopted proto- significant causes of upper GI bleeds are associated cols of management of children with upper gastrointestinal bleeding were applied at Gastroenterology & Hepatology Unit with ulcers, erosive esophagitis, gastritis, varices, of Assiut University Children Hospital. and/or Mallory-Weiss tears. While Physiologic Patients and Methods: This study is a an audit on man- stress, NSAIDs such as aspirin and ibuprofen, and agement of children with upper gastrointestinal bleeding infection with Helicobacter pylori are few of the admitted to pediatric Gastroenterology and Hepatology Unit, factors contributing to the imbalance leading to Assiut University Children Hospital during the period from ulcers and erosions in the GI tract [4] . -

Candida Esophagitis Complicated by Esophageal Stricture
E180 UCTN – Unusual cases and technical notes Candida esophagitis complicated by esophageal stricture Fig. 1 Esophageal luminal narrowing was Fig. 2 Follow-up endoscopy performed Fig. 3 Follow-up endoscopy for the evalua- observed at 23 cm from the central incisor 6 weeks after the initial evaluation at our hos- tion of dysphagia 3 months after the initiation with irregular mucosa and multiple whitish pital showed improvement of inflammation, of treatment with a antifungal agent revealed exudates, through which the scope (GIF-H260, but still the narrowed lumen did not allow the further stenosed lumen, through which not Olympus, Japan) could not pass. passage of the endoscope. even the GIF-Q260, an endoscope of smaller caliber than the GIF-H260, could pass. A 31-year-old woman was referred to the department of gastroenterology with dys- phagia accompanied by odynophagia without weight loss. The patient was immunocompetent and her only medica- tion was synthyroid, which she had been taking for the past 15 years due to hypo- thyroidism. The patient said that she had her first recurrent episodes of odynopha- gia 7 years previously and recalled that endoscopic examination at that time had revealed severe candida esophagitis. Her symptoms improved after taking medi- cation for 1 month. She was without symptoms for a couple of years, but about 5 years prior to the current presentation, Fig. 4 Barium esophagogram demonstrated narrowing of the upper and mid-esophagus (arrows) she began to experience dysphagia from with unaffected distal esophagus. time to time when taking pills or swal- lowing meat, and these episodes had be- come more frequent and had worsened cer and pseudoepitheliomatous hyperpla- the GIF-Q260 could not pass (●" Fig. -

Dyspepsia (Indigestion)
Indigestion (dydpepsia). Indigestion information - Patient | Patient Page 1 of 5 View this article online at https://patient.info/health/dyspepsia-indigestion Dyspepsia (Indigestion) Dyspepsia (indigestion) is a term which describes pain and sometimes other symptoms which come from your upper gut (the stomach, oesophagus or duodenum). There are various causes (described below). Treatment depends on the likely cause. Understanding digestion Food passes down the gullet (oesophagus) into the stomach. The stomach makes acid which is not essential but helps to digest food. Food then passes gradually into the first part of the small intestine (the duodenum). In the duodenum and the rest of the small intestine, food mixes with chemicals called enzymes. The enzymes come from the pancreas and from cells lining the intestine. The enzymes break down (digest) the food. Digested food is then absorbed into the body from the small intestine. What is dyspepsia? Dyspepsia is a term which includes a group of symptoms that come from a problem in your upper gut. The gut (gastrointestinal tract) is the tube that starts at the mouth and ends at the anus. The upper gut includes the oesophagus, stomach and duodenum. Various conditions cause dyspepsia. The main symptom is usually pain or discomfort in the upper tummy (abdomen). In addition, other symptoms that may develop include: • Bloating. • Belching. • Quickly feeling full after eating. • Feeling sick (nausea). • Being sick (vomiting). Symptoms are often related to eating. Doctors used to include heartburn (a burning sensation felt in the lower chest area) and bitter-tasting liquid coming up into the back of the throat (sometimes called 'waterbrash') as symptoms of dyspepsia. -

Hemosuccus Pancreaticus: a Rare Cause of Upper Gastrointestinal Bleeding During Pregnancy Rani Akhil Bhat,1 Vani Ramkumar,1 K
Hemosuccus Pancreaticus: A Rare Cause Of Upper Gastrointestinal Bleeding During Pregnancy Rani Akhil Bhat,1 Vani Ramkumar,1 K. Akhil Krishnanand Bhat, 2 Rajgopal Shenoy2 Abstract Upper gastrointestinal bleeding is most commonly caused by From the 1Department of Department of Obstetrics and Gynaecology, Oman Medical 2 lesions in the esophagus, stomach or duodenum. Bleeding which College, Sohar, Sultanate of Oman, Department of Surgery, Oman Medical College, Sohar, Sultanate of Oma. originates from the pancreatic duct is known as hemosuccus pancreaticus. Only a few scattered case reports of hemosuccus Received: 06 Nov 2009 pancreaticus during pregnancy have been recorded in literature. Accepted: 31 Dec 2009 This is a case of a primigravida with 37 weeks of gestation Address correspondence and reprint request to: Dr. Rani A. Bhat,Department of with hemosuccus pancreaticus and silent chronic pancreatitis. Obstetrics and Gynaecology, Oman Medical College, P. O. Box 391, P. C. 321, Al- Evaluating pregnant women with upper gastrointestinal Tareef, Sohar, Sultanate of Oman. bleeding differs from that of non pregnant women as diagnostic E-mail: [email protected] modalities using radiation cannot be used. Therefore, Esophagogastroduodenoscopy should be performed at the time of active bleeding to diagnose hemosuccus pancreaticus. Bhat RA, et al. OMJ. 25 (2010); doi:10.5001/omj.2010.21 Introduction examination showed a combination of dark red blood and melena. Laboratory investigations revealed hemoglobin of 6.3 grams/dL, Hemosuccus pancreaticus is the term used to describe the liver function tests, serum amylase, glucose and prothrombin time syndrome of gastrointestinal bleeding into the pancreatic duct were within the normal range. -

Abdominal Pain - Gastroesophageal Reflux Disease
ACS/ASE Medical Student Core Curriculum Abdominal Pain - Gastroesophageal Reflux Disease ABDOMINAL PAIN - GASTROESOPHAGEAL REFLUX DISEASE Epidemiology and Pathophysiology Gastroesophageal reflux disease (GERD) is one of the most commonly encountered benign foregut disorders. Approximately 20-40% of adults in the United States experience chronic GERD symptoms, and these rates are rising rapidly. GERD is the most common gastrointestinal-related disorder that is managed in outpatient primary care clinics. GERD is defined as a condition which develops when stomach contents reflux into the esophagus causing bothersome symptoms and/or complications. Mechanical failure of the antireflux mechanism is considered the cause of GERD. Mechanical failure can be secondary to functional defects of the lower esophageal sphincter or anatomic defects that result from a hiatal or paraesophageal hernia. These defects can include widening of the diaphragmatic hiatus, disturbance of the angle of His, loss of the gastroesophageal flap valve, displacement of lower esophageal sphincter into the chest, and/or failure of the phrenoesophageal membrane. Symptoms, however, can be accentuated by a variety of factors including dietary habits, eating behaviors, obesity, pregnancy, medications, delayed gastric emptying, altered esophageal mucosal resistance, and/or impaired esophageal clearance. Signs and Symptoms Typical GERD symptoms include heartburn, regurgitation, dysphagia, excessive eructation, and epigastric pain. Patients can also present with extra-esophageal symptoms including cough, hoarse voice, sore throat, and/or globus. GERD can present with a wide spectrum of disease severity ranging from mild, intermittent symptoms to severe, daily symptoms with associated esophageal and/or airway damage. For example, severe GERD can contribute to shortness of breath, worsening asthma, and/or recurrent aspiration pneumonia. -

Abnormal Small Bowel Permeability and Duodenitis in Recurrent Abdominal Pain
Archives ofDisease in Childhood 1990; 65: 1311-1314 1311 Abnormal small bowel permeability and duodenitis Arch Dis Child: first published as 10.1136/adc.65.12.1311 on 1 December 1990. Downloaded from in recurrent abdominal pain S B van der Meer, P P Forget, J W Arends Abstract assess the value of 5"Cr-EDTA permeability Thirty nine children with recurrent abdominal tests of intestinal inflammation. As the absorp- pain aged between 5.5 and 12 years, underwent tion of 51Cr-EDTA takes place predominantly endoscopic duodenal biopsy to find out if in the small bowel,'2 duodenal biopsies from 39 there were any duodenal inflammatory patients with recurrent abdominal pain were changes, and if there was a relationship examined for the presence of inflammatory between duodenal inflammation and intestinal changes. permeability to 5tCr-EDTA. Duodenal in- flammation was graded by the duodenitis scale of Whitehead et al (grade 0, 1, 2, and 3). Patients and methods In 13 out of 39 patients (33%) definite signs of During a prospective study 106 children with inflammation were found (grade 2 and 3). recurrent abdominal pain were investigated Intestinal permeability to 5tCr-EDTA in according to a standard protocol. Patients were patients with duodenitis (grade 1, 2, and 3) diagnosed as having recurrent abdominal pain if was significantly higher (4-42 (1-73)%) than in they were aged between 5 5 and 12 years; had patients with normal (grade 0) duodenal had recurrent abdominal pain for at least six biopsy appearances (3.3 (0-9)%). A significant months; had had attacks of pain varying in association was found between duodenal in- severity, duration, and frequency; and if their flammation and abnormal intestinal per- attacks were sometimes accompanied by pale- meability. -

~L.Il~I~1 ORGANISATION MONDIALE ORGANIZATION DE LA SANTE ~,JII /'1 'IJ,Rj ~,JII-:.CJI REGIONAL OFFICE for THE
WORLD HEALTH ~l.Il~I~1 ORGANISATION MONDIALE ORGANIZATION DE LA SANTE ~,JII /'1 'IJ,rJ ~,JII-:.CJI REGIONAL OFFICE FOR THE . BUREAU REGIONAL DE LA EASTERN MEDITBRRANEAN MEDITERRANEE ORIENTALE REGIONAL CCMVIITrEE FOR THE EM/RC14/Tech.Disc./3 EASTERN MEDITERRANEAN 25 August 1964 Fourteenth Session Agenda item 12 TECHNICAL DISCUSSIONS INFANTILE DIARRHOEA CONTRIBUTIONS FROM VARIOUS EXPERTS IN THE EASTERN MEDITERRANEAN REGION (In original language o~ presentation) EM/RC14/Tech.Disc./3 TABLE OF CONTENTS INFANTILE DIARRHOEA IN EGYPl', UAR, by A. Shafik Abbasy, M.D. 1 I Assessment of the Magnitude of Diarrhoeal Disease 1 II Study of Factors Influencing Morbidity and Mortality 3 III Etiological Studies 7 IV Conclusion 13 V Recommendations 13 References 17 INFANTILE DIARRHOEA IN EGYPl', UAR, by Dr. Girgis Abd-EI-Messih 21 I Introduction 21 II Statistical Data in Egypt 22 III Socio-Economic and Environmental Sanitary Factors in Relation to Infantile Diarrhoea 23 IV Comparison between Rates in Egypt and in some other Countries 24 V The Problem of Infantile Diarrhoea in Egypt 25 References 27 Tables 1 to 8 28 MANAGEMENT OF THE DIARRHOEAL DISEASES IN IRAN, by Dr. Hassan Ahari 35 References 42 INFAl·lrILE DIARRHOEA - Summary of Routine Care and Conclusions 43 observed at the Paediatric Ward, Pahlavi University Hospital, Teheran, by Mohammad Gharib, M.D. THE PROBLEM OF INFANTILE DIARRHOEA IN PAKISTAN, by Dr. Hamid Ali Khan 47 I Case History 48 II Degree of Dehydration 49 III Laboratory Investigation 50 IV Principles of Treatment followed 51 V Results and Analysis 53 VI Treatment and Results 56 VII Further Plans for the Study 57 L 'ETAT ACTUEL DES DIARRHEES INFANTlIES EN IRAN, par le Dr. -
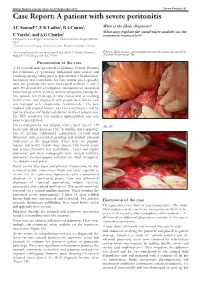
Case Report: a Patient with Severe Peritonitis
Malawi Medical Journal; 25(3): 86-87 September 2013 Severe Peritonitis 86 Case Report: A patient with severe peritonitis J C Samuel1*, E K Ludzu2, B A Cairns1, What is the likely diagnosis? 2 1 What may explain the small white nodules on the C Varela , and A G Charles transverse mesocolon? 1 Department of Surgery, University of North Carolina, Chapel Hill NC USA 2 Department of Surgery, Kamuzu Central Hospital, Lilongwe Malawi Corresponding author: [email protected] 4011 Burnett Womack Figure1. Intraoperative photograph showing the transverse mesolon Bldg CB 7228, Chapel Hill NC 27599 (1a) and the pancreas (1b). Presentation of the case A 42 year-old male presented to Kamuzu Central Hospital for evaluation of worsening abdominal pain, nausea and vomiting starting 3 days prior to presentation. On admission, his history was remarkable for four similar prior episodes over the previous five years that lasted between 3 and 5 days. He denied any constipation, obstipation or associated hematemesis, fevers, chills or urinary symptoms. During the first episode five years ago, he was evaluated at an outlying health centre and diagnosed with peptic ulcer disease and was managed with omeprazole intermittently . His past medical and surgical history was non contributory and he had no allergies and he denied alcohol intake or tobacco use. His HIV serostatus was negative approximately one year prior to presentation. On examination he was afebrile, with a heart rate of 120 (Fig 1B) beats/min, blood pressure 135/78 mmHg and respiratory rate of 22/min. Abdominal examination revealed mild distension with generalized guarding and marked rebound tenderness in the epigastrium. -

Gastritis - Symptoms and Causes - Mayo Clinic Visited 11/22/2019
Gastritis - Symptoms and causes - Mayo Clinic Visited 11/22/2019 Request an Appointment Find a Doctor MENU Find a Job Give Now Log in to Patient Account English Patient Care & Health Information Diseases & Conditions Request an Gastritis Appointment Symptoms & causes Diagnosis & treatment Doctors & departments Overview Print Advertisement Gastritis is a general term for a group Mayo Clinic does not endorse companies or of conditions with one thing in common: products. Advertising revenue supports our not- inflammation of the lining of the for-profit mission. stomach. The inflammation of gastritis Advertising & Sponsorship is most often the result of infection with Policy Opportunities Ad Choices the same bacterium that causes most stomach ulcers. Regular use of certain Stomach and pain relievers and drinking too much pyloric valve Mayo Clinic Marketplace alcohol also can contribute to gastritis. Check out these best-sellers and special offers on books and newsletters from Mayo Clinic. Gastritis may occur suddenly (acute gastritis), or appear slowly over time (chronic gastritis). In some cases, gastritis NEW – Guide to Fibromyalgia can lead to ulcers and an increased risk of stomach cancer. Instant access – Mayo Clinic Health Letter For most people, however, gastritis isn't serious and improves quickly with treatment. Diabetes? This diet works … Stop osteoporosis in its tracks The Mayo Clinic Diet Online Products & Services Book: Mayo Clinic on Digestive Health https://www.mayoclinic.org/diseases-conditions/gastritis/symptoms-causes/syc-20355807[11/22/2019 4:16:25 PM] Gastritis - Symptoms and causes - Mayo Clinic Visited 11/22/2019 Symptoms The signs and symptoms of gastritis include: Gnawing or burning ache or pain (indigestion) in your upper abdomen that may become either worse or better with eating Nausea Vomiting A feeling of fullness in your upper abdomen after eating Gastritis doesn't always cause signs and symptoms. -

Etiology of Upper Gastrointestinal Haemorrhage in a Teaching Hospital
TAJ June 2008; Volume 21 Number 1 ISSN 1019-8555 The Journal of Teachers Association RMC, Rajshahi Original Article Etiology of Upper Gastrointestinal Haemorrhage in a Teaching Hospital M Uddin Ahmed1, M Abdul Ahad2, M A Alim2, A R M Saifuddin Ekram3, Q Abdullah Al Masum4, Sumona Tanu5, Refaz Uddin6 Abstract A descriptive study on all cases of haematemesis and or melaena was carried out at Rajshahi Medical College Hospital to observe the demographic profile, clinical presentation, cause and outcome of upper gastrointestinal bleeding in a tertiary hospital of Bangladesh. Fifty adult patients presenting with haematemesis and or melaena admitted consecutively into medical unit were evaluated through proper history taking, thorough clinical examination, endoscopic examination with in 48 hours of first presentation and other related investigations. Patients those who were not stabilized haemodynamically with in 48 hours of resuscitation and endoscopy could not be done with in that period were excluded from this study. Results our results showed that out of 50 patients 44 were male and 6 were female and average age of the patients was 39.9 years. Most of the patients were from low socio-economic condition. Farmers, service holders and laborers were the most (57%) affected group. Haematemesis and melaena (42%), only melaena (42%) and only haematemesis (16%) were the presenting features. Endoscopy revealed that duodenal ulcer( 34%) was the most common cause of UGI bleeding followed by rupture of portal varices( 16%) , neoplasm( 10%) , gastric ulcer ( 08%) and gastric erosion( 06%). Acute upper GI bleeding is a common medical problem that is responsible for significant morbidity and mortality. -
Anatomy of Small Intestine Doctors Notes Notes/Extra Explanation Please View Our Editing File Before Studying This Lecture to Check for Any Changes
Color Code Important Anatomy of Small Intestine Doctors Notes Notes/Extra explanation Please view our Editing File before studying this lecture to check for any changes. Objectives: At the end of the lecture, students should: List the different parts of small intestine. Describe the anatomy of duodenum, jejunum & ileum regarding: the shape, length, site of beginning & termination, peritoneal covering, arterial supply & lymphatic drainage. Differentiate between each part of duodenum regarding the length, level & relations. Differentiate between the jejunum & ileum regarding the characteristic anatomical features of each of them. Abdomen What is Mesentery? It is a double layer attach the intestine to abdominal wall. If it has mesentery it is freely moveable. L= liver, S=Spleen, SI=Small Intestine, AC=Ascending Colon, TC=Transverse Colon Abdomen The small intestines consist of two parts: 1- fixed part (no mesentery) (retroperitoneal) : duodenum 2- free (movable) part (with mesentery) :jejunum & ileum Only on the boys’ slides RELATION BETWEEN EMBRYOLOGICAL ORIGIN & ARTERIAL SUPPLY مهم :Extra Arterial supply depends on the embryological origin : Foregut Coeliac trunk Midgut superior mesenteric Hindgut Inferior mesenteric Duodenum: • Origin: foregut & midgut • Arterial supply: 1. Coeliac trunk (artery of foregut) 2. Superior mesenteric: (artery of midgut) The duodenum has 2 arterial supply because of the double origin The junction of foregut and midgut is at the second part of the duodenum Jejunum & ileum: • Origin: midgut • Arterial -

Obscure Gastrointestinal Bleeding in Cirrhosis: Work-Up and Management
Current Hepatology Reports (2019) 18:81–86 https://doi.org/10.1007/s11901-019-00452-6 MANAGEMENT OF CIRRHOTIC PATIENT (A CARDENAS AND P TANDON, SECTION EDITORS) Obscure Gastrointestinal Bleeding in Cirrhosis: Work-up and Management Sergio Zepeda-Gómez1 & Brendan Halloran1 Published online: 12 February 2019 # Springer Science+Business Media, LLC, part of Springer Nature 2019 Abstract Purpose of Review Obscure gastrointestinal bleeding (OGIB) in patients with cirrhosis can be a diagnostic and therapeutic challenge. Recent advances in the approach and management of this group of patients can help to identify the source of bleeding. While the work-up of patients with cirrhosis and OGIB is the same as with patients without cirrhosis, clinicians must be aware that there are conditions exclusive for patients with portal hypertension that can potentially cause OGIB. Recent Findings New endoscopic and imaging techniques are capable to identify sources of OGIB. Balloon-assisted enteroscopy (BAE) allows direct examination of the small-bowel mucosa and deliver specific endoscopic therapy. Conditions such as ectopic varices and portal hypertensive enteropathy are better characterized with the improvement in visualization by these techniques. New algorithms in the approach and management of these patients have been proposed. Summary There are new strategies for the approach and management of patients with cirrhosis and OGIB due to new develop- ments in endoscopic techniques for direct visualization of the small bowel along with the capability of endoscopic treatment for different types of lesions. Patients with cirrhosis may present with OGIB secondary to conditions associated with portal hypertension. Keywords Obscure gastrointestinal bleeding . Cirrhosis . Portal hypertension .