18 EDX Studies in ALS and Other Motor Neuron Disorders.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Clinically Undetected Motor Neuron Disease in Pathologically Proven Frontotemporal Lobar Degeneration with Motor Neuron Disease
ORIGINAL CONTRIBUTION Clinically Undetected Motor Neuron Disease in Pathologically Proven Frontotemporal Lobar Degeneration With Motor Neuron Disease Keith A. Josephs, MST, MD; Joseph E. Parisi, MD; David S. Knopman, MD; Bradley F. Boeve, MD; Ronald C. Petersen, MD, PhD; Dennis W. Dickson, MD Background: Frontotemporal lobar degeneration with evidence of motor neuron disease. Semiquantitative motor neuron disease (FTLD-MND) is a pathological analysis of motor and extramotor pathological findings entity characterized by motor neuron degeneration and revealed a spectrum of pathological changes underlying frontotemporal lobar degeneration. The ability to detect FTLD-MND. Hippocampal sclerosis, predominantly of the clinical signs of dementia and motor neuron disease the subiculum, was a significantly more frequent occur- in pathologically confirmed FTLD-MND has not been rence in the cases without clinical evidence of motor assessed. neuron disease (PϽ.01). In addition, neuronal loss, gliosis, and corticospinal tract degeneration were less Objectives: To determine if all cases of pathologically severe in the other 3 cases without clinical evidence of confirmed FTLD-MND have clinical evidence of fronto- motor neuron disease. temporal dementia and motor neuron disease, and to de- termine the possible reasons for misdiagnosis. Conclusions: Clinical diagnostic sensitivity for the el- ements of FTLD-MND is modest and may be affected by Method: Review of historical records and semiquantita- the fact that FTLD-MND represents a spectrum of patho- tive analysis of the motor and extramotor pathological find- logical findings, rather than a single homogeneous en- ings of all cases of pathologically confirmed FTLD-MND. tity. Detection of signs of clinical motor neuron disease is also difficult when motor neuron degeneration is mild Results: From a total of 17 cases of pathologically con- and in patients with hippocampal sclerosis. -

Primary Lateral Sclerosis, Upper Motor Neuron Dominant Amyotrophic Lateral Sclerosis, and Hereditary Spastic Paraplegia
brain sciences Review Upper Motor Neuron Disorders: Primary Lateral Sclerosis, Upper Motor Neuron Dominant Amyotrophic Lateral Sclerosis, and Hereditary Spastic Paraplegia Timothy Fullam and Jeffrey Statland * Department of Neurology, University of Kansas Medical Center, Kansas, KS 66160, USA; [email protected] * Correspondence: [email protected] Abstract: Following the exclusion of potentially reversible causes, the differential for those patients presenting with a predominant upper motor neuron syndrome includes primary lateral sclerosis (PLS), hereditary spastic paraplegia (HSP), or upper motor neuron dominant ALS (UMNdALS). Differentiation of these disorders in the early phases of disease remains challenging. While no single clinical or diagnostic tests is specific, there are several developing biomarkers and neuroimaging technologies which may help distinguish PLS from HSP and UMNdALS. Recent consensus diagnostic criteria and use of evolving technologies will allow more precise delineation of PLS from other upper motor neuron disorders and aid in the targeting of potentially disease-modifying therapeutics. Keywords: primary lateral sclerosis; amyotrophic lateral sclerosis; hereditary spastic paraplegia Citation: Fullam, T.; Statland, J. Upper Motor Neuron Disorders: Primary Lateral Sclerosis, Upper 1. Introduction Motor Neuron Dominant Jean-Martin Charcot (1825–1893) and Wilhelm Erb (1840–1921) are credited with first Amyotrophic Lateral Sclerosis, and describing a distinct clinical syndrome of upper motor neuron (UMN) tract degeneration in Hereditary Spastic Paraplegia. Brain isolation with symptoms including spasticity, hyperreflexia, and mild weakness [1,2]. Many Sci. 2021, 11, 611. https:// of the earliest described cases included cases of hereditary spastic paraplegia, amyotrophic doi.org/10.3390/brainsci11050611 lateral sclerosis, and underrecognized structural, infectious, or inflammatory etiologies for upper motor neuron dysfunction which have since become routinely diagnosed with the Academic Editors: P. -

Acute Limb Ischemia Secondary to Myositis- Induced Compartment Syndrome in a Patient with Human Immunodeficiency Virus Infection
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Acute limb ischemia secondary to myositis- induced compartment syndrome in a patient with human immunodeficiency virus infection Russell Lam, MD, Peter H. Lin, MD, Suresh Alankar, MD, Qizhi Yao, MD, PhD, Ruth L. Bush, MD, Changyi Chen, MD, PhD, and Alan B. Lumsden, MD, Houston, Tex Myositis, while uncommon, develops more frequently in patients with human immunodeficiency virus infection. We report a case of acute lower leg ischemia caused by myositis in such a patient. Urgent four-compartment fasciotomy of the lower leg was performed, which decompressed the compartmental hypertension and reversed the arterial ischemia. This case underscores the importance of recognizing compartment syndrome as a cause of acute limb ischemia. (J Vasc Surg 2003;37:1103-5.) Compartment syndrome results from elevated pressure compartment was firm and tender. Additional pertinent laboratory within an enclosed fascial space, which can occur after studies revealed creatine phosphokinase level of 53,350 U/L; fracture, soft tissue injury, or reperfusion after arterial isch- serum creatinine concentration had increased to 3.5 mg/dL, and emia.1 Other less common causes of compartment syn- WBC count had increased to 18,000 cells/mm3. Venous duplex drome include prolonged limb compression, burns, and scans showed no evidence of deep venous thrombosis in the right extreme exertion.1 Soft tissue infection in the form of lower leg. Pressure was measured in all four compartments of the myositis is a rare cause of compartment syndrome. We right calf and ranged from 55 to 65 mm Hg. -
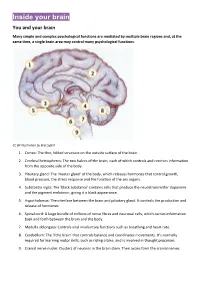
Inside Your Brain You and Your Brain
Inside your brain You and your brain Many simple and complex psychological functions are mediated by multiple brain regions and, at the same time, a single brain area may control many psychological functions. CC BY Illustration by Bret Syfert 1. Cortex: The thin, folded structure on the outside surface of the brain. 2. Cerebral hemispheres: The two halves of the brain, each of which controls and receives information from the opposite side of the body. 3. Pituitary gland: The ‘master gland’ of the body, which releases hormones that control growth, blood pressure, the stress response and the function of the sex organs. 4. Substantia nigra: The ‘black substance’ contains cells that produce the neurotransmitter dopamine and the pigment melatonin, giving it a black appearance. 5. Hypothalamus: The interface between the brain and pituitary gland. It controls the production and release of hormones. 6. Spinal cord: A large bundle of millions of nerve fibres and neuronal cells, which carries information back and forth between the brain and the body. 7. Medulla oblongata: Controls vital involuntary functions such as breathing and heart rate. 8. Cerebellum: The ‘little brain’ that controls balance and coordinates movements. It’s normally required for learning motor skills, such as riding a bike, and is involved in thought processes. 9. Cranial nerve nuclei: Clusters of neurons in the brain stem. Their axons form the cranial nerves. Your brain underpins who you are. It stores your knowledge and memories, gives you the capacity for thought and emotion, and enables you to control your body. The brain is just one part of the nervous system. -

Management of Neurogenic Dysphagia
694 Postgrad Med J 2001;77:694–699 Postgrad Med J: first published as 10.1136/pmj.77.913.694 on 1 November 2001. Downloaded from Management of neurogenic dysphagia A M O Bakheit Dysphagia is common in patients with neuro- of the cerebral cortex, basal ganglia, brain logical disorders. It may result from lesions in stem, cerebellum, and lower cranial nerves may the central or peripheral nervous system as well result in dysphagia. Degeneration of the as from diseases of muscle and disorders of the myenteric ganglion cells in the oesophagus, neuromuscular junction. Drugs that are com- muscle diseases and disorders of neuromusc- monly used in the management of neurological ular transmission, for example myasthenia conditions may also precipitate or aggravate gravis and Eaton-Lambers syndrome, are other swallowing diYculties in some patients. Neuro- less common causes. genic dysphagia often results in serious compli- cations, including pulmonary aspiration, dehy- CEREBRAL CORTEX dration, and malnutrition. These The commonest condition associated with complications are usually preventable if the dysphagia resulting from cortical lesions is stroke. Acute stroke is complicated by dys- dysphagia is recognised early and managed 1 appropriately. phagia in about 25%–42% of all cases. Dysphagia in these patients is usually associ- Physiological mechanisms of neurogenic ated with hemiplegia due to lesions of the brain stem or the involvement of one or both dysphagia The act of swallowing may be viewed as three hemispheres. However, on rare occasions, dys- discrete but inter-related physiological stages: phagia may be the sole manifestation of a cer- the oral, pharyngeal, and oesophageal phases. -

Management of Postpolio Syndrome
Review Management of postpolio syndrome Henrik Gonzalez, Tomas Olsson, Kristian Borg Lancet Neurol 2010; 9: 634–42 Postpolio syndrome is characterised by the exacerbation of existing or new health problems, most often muscle weakness See Refl ection and Reaction and fatigability, general fatigue, and pain, after a period of stability subsequent to acute polio infection. Diagnosis is page 561 based on the presence of a lower motor neuron disorder that is supported by neurophysiological fi ndings, with exclusion Division of Rehabilitation of other disorders as causes of the new symptoms. The muscle-related eff ects of postpolio syndrome are possibly Medicine, Department of associated with an ongoing process of denervation and reinnervation, reaching a point at which denervation is no Clinical Sciences, Danderyd Hospital (H Gonzalez MD, longer compensated for by reinnervation. The cause of this denervation is unknown, but an infl ammatory process is K Borg MD) and Department of possible. Rehabilitation in patients with postpolio syndrome should take a multiprofessional and multidisciplinary Clinical Neurosciences, Centre approach, with an emphasis on physiotherapy, including enhanced or individually modifi ed physical activity, and muscle for Molecular Medicine training. Patients with postpolio syndrome should be advised to avoid both inactivity and overuse of weak muscles. (T Olsson MD), Karolinska Institute, Stockholm, Sweden Evaluation of the need for orthoses and assistive devices is often required. Correspondence to: Henrik Gonzalez, Division of Introduction summary of the pathophysiology and clinical Rehabilitation Medicine, 12–20 million people worldwide have sequelae of characteristics of postpolio syndrome, outline diagnostic Department of Clinical Sciences, poliomyelitis, according to Post-Polio Health and treatment options, and suggest future research Karolinska Institute, Danderyd Hospital, S-182 88 Stockholm, International. -

ALS and Other Motor Neuron Diseases Can Represent Diagnostic Challenges
Review Article Address correspondence to Dr Ezgi Tiryaki, Hennepin ALS and Other Motor County Medical Center, Department of Neurology, 701 Park Avenue P5-200, Neuron Diseases Minneapolis, MN 55415, [email protected]. Ezgi Tiryaki, MD; Holli A. Horak, MD, FAAN Relationship Disclosure: Dr Tiryaki’s institution receives support from The ALS Association. Dr Horak’s ABSTRACT institution receives a grant from the Centers for Disease Purpose of Review: This review describes the most common motor neuron disease, Control and Prevention. ALS. It discusses the diagnosis and evaluation of ALS and the current understanding of its Unlabeled Use of pathophysiology, including new genetic underpinnings of the disease. This article also Products/Investigational covers other motor neuron diseases, reviews how to distinguish them from ALS, and Use Disclosure: Drs Tiryaki and Horak discuss discusses their pathophysiology. the unlabeled use of various Recent Findings: In this article, the spectrum of cognitive involvement in ALS, new concepts drugs for the symptomatic about protein synthesis pathology in the etiology of ALS, and new genetic associations will be management of ALS. * 2014, American Academy covered. This concept has changed over the past 3 to 4 years with the discovery of new of Neurology. genes and genetic processes that may trigger the disease. As of 2014, two-thirds of familial ALS and 10% of sporadic ALS can be explained by genetics. TAR DNA binding protein 43 kDa (TDP-43), for instance, has been shown to cause frontotemporal dementia as well as some cases of familial ALS, and is associated with frontotemporal dysfunction in ALS. Summary: The anterior horn cells control all voluntary movement: motor activity, res- piratory, speech, and swallowing functions are dependent upon signals from the anterior horn cells. -

Approach to a Patient with Hemiplegia and Monoplegia
CHAPTER Approach to a Patient with Hemiplegia and Monoplegia 27 Sudhir Kumar, Subhash Kaul INTRODUCTION 4. Injury to multiple cervical nerve roots. Monoplegia and hemiplegia are common neurological 5. Functional or psychogenic. symptoms in patients presenting to the emergency department as well as outpatient department. Insidious onset, gradually progressive monoplegia affecting lower limb can be caused by the following Monoplegia refers to weakness of one limb (either arm or conditions: leg) and hemiplegia refers to weakness of one arm and leg on the same side of body (either left or right side). 1. Tumor of the contralateral frontal lobe. There are a variety of underlying causes for monoplegia 2. Tumor of spinal cord at thoracic or lumbar level. and hemiplegia. The causes differ in different age groups. 3. Chronic infection of brain (frontal lobe) or spinal The causes also differ depending on the onset, progression cord (thoracic or lumbar level), such as tuberculous. and duration of weakness. Therefore, one needs to adopt a systematic approach during history taking and 4. Lumbosacral-plexopathy, due to diabetes mellitus. examination in order to arrive at the correct diagnosis. Insidious onset, gradually progressive monoplegia, Appropriate investigations after these would confirm the affecting upper limb, can be caused by one of the following diagnosis. conditions: The aim of this chapter is to systematically look at the 1. Tumor of the contralateral parietal lobe. differential diagnosis of monoplegia and hemiplegia and outline the approach needed to pinpoint the exact 2. Compressive lesion (tumor, large disc, etc) in underlying cause. cervical cord region. 3. Chronic infection of the brain (parietal lobe) or APPROACH TO THE DIAGNOSIS OF MONOPLEGIA spinal cord (cervical region), such as tuberculous. -

Neuromuscular Disorders Neurology in Practice: Series Editors: Robert A
Neuromuscular Disorders neurology in practice: series editors: robert a. gross, department of neurology, university of rochester medical center, rochester, ny, usa jonathan w. mink, department of neurology, university of rochester medical center,rochester, ny, usa Neuromuscular Disorders edited by Rabi N. Tawil, MD Professor of Neurology University of Rochester Medical Center Rochester, NY, USA Shannon Venance, MD, PhD, FRCPCP Associate Professor of Neurology The University of Western Ontario London, Ontario, Canada A John Wiley & Sons, Ltd., Publication This edition fi rst published 2011, ® 2011 by Blackwell Publishing Ltd Blackwell Publishing was acquired by John Wiley & Sons in February 2007. Blackwell’s publishing program has been merged with Wiley’s global Scientifi c, Technical and Medical business to form Wiley-Blackwell. Registered offi ce: John Wiley & Sons Ltd, The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, UK Editorial offi ces: 9600 Garsington Road, Oxford, OX4 2DQ, UK The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, UK 111 River Street, Hoboken, NJ 07030-5774, USA For details of our global editorial offi ces, for customer services and for information about how to apply for permission to reuse the copyright material in this book please see our website at www.wiley.com/wiley-blackwell The right of the author to be identifi ed as the author of this work has been asserted in accordance with the UK Copyright, Designs and Patents Act 1988. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, except as permitted by the UK Copyright, Designs and Patents Act 1988, without the prior permission of the publisher. -

Motor Neuron Disease and the Elderly
Neurology 61 Motor neuron disease and the elderly Motor neuron disease is a devastating condition characterised by degeneration of motor nerves. Many of the presenting symptoms, such as fatigue, muscle weakness and difficulty in swallowing have a broad differential diagnoses in the elderly population. Dr Sheba Azam and Professor PN Leigh explain how ensuring quality of life for patients requires preventing unnecessary delay in diagnosis and early referral to an appropriate multidisciplinary team. myotrophic lateral sclerosis (ALS) also cognitive changes. Thus, misdiagnosis as well as known as motor neuron disease (MND) under-investigation has been suggested as possible (the terms are used interchangeably), was causes of an apparent decrease in the incidence of A 4 fi rst described in 1869 by the French neurologist MND in later life . Jean-Martin Charcot1. It is a progressive, fatal neurological disease characterised by degeneration of motor nerve cells in the motor cortex, Prognostic factors corticospinal tract and the spinal cord anterior horn Although the average survival in MND is around cells. The degeneration of motor nerve cells results 36 months, some patients live for 10 years or more. in progressive muscle wasting leading to signifi cant Certain phenotypic variants appear to determine disability and ultimately death. Death usually survival rates. Using information held in a tertiary results from respiratory failure due to weakness of referral MND database, a group of researchers the respiratory muscles. analysed data on onset of disease, site of onset and duration of survival5. The authors concluded that typical MND with bulbar onset, onset later in life Incidence and prevalence or in the defi nite category of El Escorial (where The worldwide incidence of MND is approximately the World Federation of Neurologist meet to a professor of clinical two per 100,000 and the prevalence is four to seven decide diagnostic criteria) at presentation, per 100,000. -

ICD9 & ICD10 Neuromuscular Codes
ICD-9-CM and ICD-10-CM NEUROMUSCULAR DIAGNOSIS CODES ICD-9-CM ICD-10-CM Focal Neuropathy Mononeuropathy G56.00 Carpal tunnel syndrome, unspecified Carpal tunnel syndrome 354.00 G56.00 upper limb Other lesions of median nerve, Other median nerve lesion 354.10 G56.10 unspecified upper limb Lesion of ulnar nerve, unspecified Lesion of ulnar nerve 354.20 G56.20 upper limb Lesion of radial nerve, unspecified Lesion of radial nerve 354.30 G56.30 upper limb Lesion of sciatic nerve, unspecified Sciatic nerve lesion (Piriformis syndrome) 355.00 G57.00 lower limb Meralgia paresthetica, unspecified Meralgia paresthetica 355.10 G57.10 lower limb Lesion of lateral popiteal nerve, Peroneal nerve (lesion of lateral popiteal nerve) 355.30 G57.30 unspecified lower limb Tarsal tunnel syndrome, unspecified Tarsal tunnel syndrome 355.50 G57.50 lower limb Plexus Brachial plexus lesion 353.00 Brachial plexus disorders G54.0 Brachial neuralgia (or radiculitis NOS) 723.40 Radiculopathy, cervical region M54.12 Radiculopathy, cervicothoracic region M54.13 Thoracic outlet syndrome (Thoracic root Thoracic root disorders, not elsewhere 353.00 G54.3 lesions, not elsewhere classified) classified Lumbosacral plexus lesion 353.10 Lumbosacral plexus disorders G54.1 Neuralgic amyotrophy 353.50 Neuralgic amyotrophy G54.5 Root Cervical radiculopathy (Intervertebral disc Cervical disc disorder with myelopathy, 722.71 M50.00 disorder with myelopathy, cervical region) unspecified cervical region Lumbosacral root lesions (Degeneration of Other intervertebral disc degeneration, -

Evaluation of Suspected Malignant Hyperthermia Events During Anesthesia Frank Schuster*, Stephan Johannsen, Daniel Schneiderbanger and Norbert Roewer
Schuster et al. BMC Anesthesiology 2013, 13:24 http://www.biomedcentral.com/1471-2253/13/24 RESEARCH ARTICLE Open Access Evaluation of suspected malignant hyperthermia events during anesthesia Frank Schuster*, Stephan Johannsen, Daniel Schneiderbanger and Norbert Roewer Abstract Background: Malignant hyperthermia (MH), a metabolic myopathy triggered by volatile anesthetics and depolarizing muscle relaxants, is a potentially lethal complication of general anesthesia in susceptible patients. The implementation of modern inhalation anesthetics that research indicates as less potent trigger substances and the recommended limitations of succinylcholine use, suggests there may be considerable decline of fulminant MH cases. In the presented study, the authors analyzed suspected MH episodes during general anesthesia of patients that were referred to the Wuerzburg MH unit between 2007 and 2011, assuming that MH is still a relevant anesthetic problem in our days. Methods: With approval of the local ethics committee data of patients that underwent muscle biopsy and in vitro contracture test (IVCT) between 2007 and 2011 were analyzed. Only patients with a history of suspected MH crisis were included in the study. The incidents were evaluated retrospectively using anesthetic documentation and medical records. Results: Between 2007 and 2011 a total of 124 patients were tested. 19 of them were referred because of suspected MH events; 7 patients were diagnosed MH-susceptible, 4 MH-equivocal and 8 MH-non-susceptible by IVCT. In a majority of cases masseter spasm after succinylcholine had been the primary symptom. Cardiac arrhythmias and hypercapnia frequently occurred early in the course of events. Interestingly, dantrolene treatment was initiated in a few cases only.