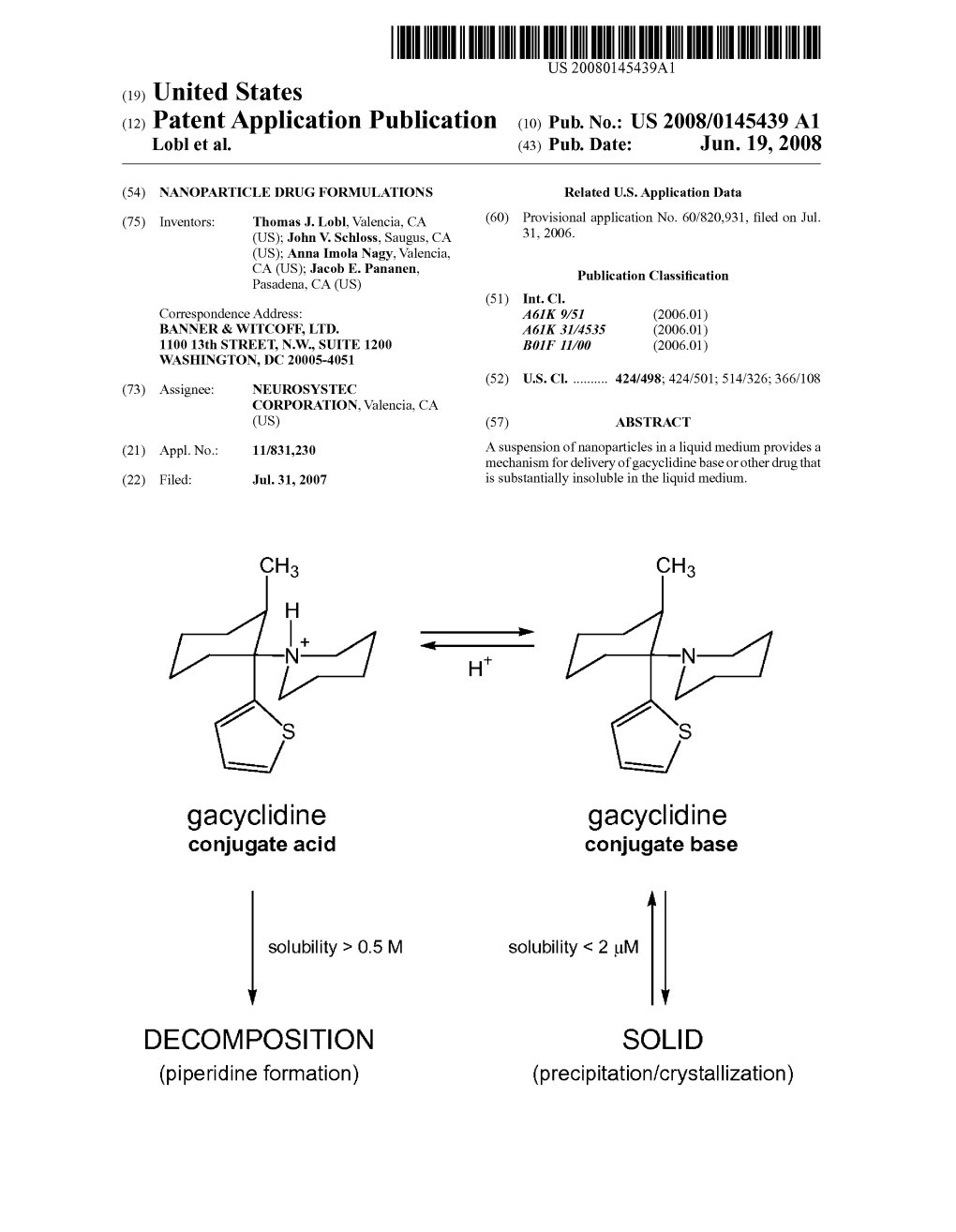
Gacyclidine Gacyclidine Conjugate Acid Conjugate Base
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Anti-Inflammatory Effects of Amantadine and Memantine
Journal of Personalized Medicine Communication Anti-Inflammatory Effects of Amantadine and Memantine: Possible Therapeutics for the Treatment of Covid-19? Félix Javier Jiménez-Jiménez 1,* , Hortensia Alonso-Navarro 1 , Elena García-Martín 2 and José A. G. Agúndez 2 1 Section of Neurology, Hospital Universitario del Sureste, Arganda del Rey, E-28500 Madrid, Spain; [email protected] 2 University Institute of Molecular Pathology Biomarkers, UNEx. ARADyAL Instituto de Salud Carlos III, E-10071 Cáceres, Spain; [email protected] (E.G.-M.); [email protected] (J.A.G.A.) * Correspondence: [email protected]; Tel.: +34-636968395 Received: 2 October 2020; Accepted: 6 November 2020; Published: 9 November 2020 Abstract: We have reviewed current data on the anti-inflammatory effects of amantadine and memantine in clinical and in vivo models of inflammation, and we propose that these effects have potential interest for the treatment of the SARS-CoV-2 infection (COVID-19 disease). To that end, we performed a literature search using the PubMed Database from 1966 up to October 31 2020, crossing the terms “amantadine” and “memantine” with “inflammation” and “anti-inflammatory”. Amantadine and/or memantine have shown anti-inflammatory effects in chronic hepatitis C, in neuroinflammation induced by sepsis and by lipopolysaccharides, experimental models of multiple sclerosis, spinal cord injury, and respiratory diseases. Since the inflammatory response is one of the main pathogenetic mechanisms in the progression of the SARS-CoV-2 infection, anti-inflammatory effects of amantadine and memantine could be hypothetically useful in the treatment of this condition. This potential utility deserves further research. Keywords: amantadine; memantine; anti-inflammatory effects; SARS-Cov-2; COVID-19; therapy 1. -

Tinnitus Hopes Put to Sleep by Latest Auris Failure
March 14, 2018 Tinnitus hopes put to sleep by latest Auris failure Madeleine Armstrong Ketamine is best known as a horse tranquiliser or a recreational drug, but it has also been proposed as a treatment for various disorders from depression to Alzheimer’s. Hopes of developing the drug in tinnitus have been dashed by the failure of Auris’s Keyzilen in a second phase III trial. As well as leaving Auris’s future looking bleak – Keyzilen is the second of its phase III candidates to flunk in four months – the setback could also be bad news for the sparse tinnitus pipeline. According to EvaluatePharma there is only one other candidate in active clinical development, Otonomy’s OTO-313, and this uses the same mechanism of action as Keyzilen (see table below). Tinnitus pipeline Generic Project Company Pharma class Trial(s) Notes name Phase III Auris Esketamine TACTT2 Keyzilen NMDA antagonist Failed Aug 2016 Medical hydrochloride (NCT01803646) TACTT3 Failed Mar 2018 (NCT02040194) Phase I OTO- Phase I/II trial to OTO-311 abandoned in Otonomy NMDA antagonist Gacyclidine 313 start H1 2019 favour of this formulation Preclinical Auris AM-102 Undisclosed - - Medical KCNQ2 Knopp Kv7 potassium - - Program Biosciences channel modulator Source: EvaluatePharma. Both projects are psychoactive drugs targeting the NMDA receptor. Tinnitus is commonly caused by loud noise and resulting damage to the sensory hair cells in the cochlea. Initial trauma to the inner ear has been shown to trigger excess production of glutamate, which leads to the hyperactivation of NMDA receptors and, in turn, cell death. Blocking the NMDA receptor could therefore have a protective effect – but it is unclear how this mechanism would work once the damage to hair cells had been done. -

(19) United States (12) Patent Application Publication (10) Pub
US 20130289061A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0289061 A1 Bhide et al. (43) Pub. Date: Oct. 31, 2013 (54) METHODS AND COMPOSITIONS TO Publication Classi?cation PREVENT ADDICTION (51) Int. Cl. (71) Applicant: The General Hospital Corporation, A61K 31/485 (2006-01) Boston’ MA (Us) A61K 31/4458 (2006.01) (52) U.S. Cl. (72) Inventors: Pradeep G. Bhide; Peabody, MA (US); CPC """"" " A61K31/485 (201301); ‘4161223011? Jmm‘“ Zhu’ Ansm’ MA. (Us); USPC ......... .. 514/282; 514/317; 514/654; 514/618; Thomas J. Spencer; Carhsle; MA (US); 514/279 Joseph Biederman; Brookline; MA (Us) (57) ABSTRACT Disclosed herein is a method of reducing or preventing the development of aversion to a CNS stimulant in a subject (21) App1_ NO_; 13/924,815 comprising; administering a therapeutic amount of the neu rological stimulant and administering an antagonist of the kappa opioid receptor; to thereby reduce or prevent the devel - . opment of aversion to the CNS stimulant in the subject. Also (22) Flled' Jun‘ 24’ 2013 disclosed is a method of reducing or preventing the develop ment of addiction to a CNS stimulant in a subj ect; comprising; _ _ administering the CNS stimulant and administering a mu Related U‘s‘ Apphcatlon Data opioid receptor antagonist to thereby reduce or prevent the (63) Continuation of application NO 13/389,959, ?led on development of addiction to the CNS stimulant in the subject. Apt 27’ 2012’ ?led as application NO_ PCT/US2010/ Also disclosed are pharmaceutical compositions comprising 045486 on Aug' 13 2010' a central nervous system stimulant and an opioid receptor ’ antagonist. -

Salicylate Induces Tinnitus Through Activation of Cochlear NMDA Receptors
3944 • The Journal of Neuroscience, May 1, 2003 • 23(9):3944–3952 Salicylate Induces Tinnitus through Activation of Cochlear NMDA Receptors Matthieu J. Guitton,1 Jean Caston,2 Je´roˆme Ruel,1 Randolph M. Johnson,3 Re´my Pujol,1 and Jean-Luc Puel1 1Institut National de la Sante´ et de la Recherche Me´dicale UR-254, Laboratoire de Neurobiologie de l’Audition–Plasticite´ Synaptique, Faculte´deMe´decine, Universite´ de Montpellier 1, 34090 Montpellier, France, 2 Unite´ Propre de Recherche et de l’Enseignement Supe´rieur 1780, Laboratoire de Neurobiologie de l’Apprentissage, Universite´ de Rouen, 76821 Mont-Saint-Aignan, France, and 3DURECT Corporation, Cupertino, California 95014 Salicylate, the active component of aspirin, is known to induce tinnitus. However, the site and the mechanism of generation of tinnitus induced by salicylate remains unclear. Here, we developed a behavioral procedure to measure tinnitus in rats. The behavioral model was based on an active avoidance paradigm in which rats had to display a motor task (i.e., to jump on a climbing pole when hearing a sound). Giving salicylate led to a decrease in the percentage of correct responses (score) and a drastic increase in the number of false positive responses (i.e., animals execute the motor task during a silent period). Presentation of the sound at a constant perceptive level prevents decrease of the score, leading to the proposal that score is related to hearing performance. In contrast, the increase of false positive responses remained unchanged. In fact, animals behaved as if they hear a sound, indicating that they are experiencing tinnitus. -

Customs Tariff - Schedule
CUSTOMS TARIFF - SCHEDULE 99 - i Chapter 99 SPECIAL CLASSIFICATION PROVISIONS - COMMERCIAL Notes. 1. The provisions of this Chapter are not subject to the rule of specificity in General Interpretative Rule 3 (a). 2. Goods which may be classified under the provisions of Chapter 99, if also eligible for classification under the provisions of Chapter 98, shall be classified in Chapter 98. 3. Goods may be classified under a tariff item in this Chapter and be entitled to the Most-Favoured-Nation Tariff or a preferential tariff rate of customs duty under this Chapter that applies to those goods according to the tariff treatment applicable to their country of origin only after classification under a tariff item in Chapters 1 to 97 has been determined and the conditions of any Chapter 99 provision and any applicable regulations or orders in relation thereto have been met. 4. The words and expressions used in this Chapter have the same meaning as in Chapters 1 to 97. Issued January 1, 2020 99 - 1 CUSTOMS TARIFF - SCHEDULE Tariff Unit of MFN Applicable SS Description of Goods Item Meas. Tariff Preferential Tariffs 9901.00.00 Articles and materials for use in the manufacture or repair of the Free CCCT, LDCT, GPT, UST, following to be employed in commercial fishing or the commercial MT, MUST, CIAT, CT, harvesting of marine plants: CRT, IT, NT, SLT, PT, COLT, JT, PAT, HNT, Artificial bait; KRT, CEUT, UAT, CPTPT: Free Carapace measures; Cordage, fishing lines (including marlines), rope and twine, of a circumference not exceeding 38 mm; Devices for keeping nets open; Fish hooks; Fishing nets and netting; Jiggers; Line floats; Lobster traps; Lures; Marker buoys of any material excluding wood; Net floats; Scallop drag nets; Spat collectors and collector holders; Swivels. -

4 Supplementary File
Supplemental Material for High-throughput screening discovers anti-fibrotic properties of Haloperidol by hindering myofibroblast activation Michael Rehman1, Simone Vodret1, Luca Braga2, Corrado Guarnaccia3, Fulvio Celsi4, Giulia Rossetti5, Valentina Martinelli2, Tiziana Battini1, Carlin Long2, Kristina Vukusic1, Tea Kocijan1, Chiara Collesi2,6, Nadja Ring1, Natasa Skoko3, Mauro Giacca2,6, Giannino Del Sal7,8, Marco Confalonieri6, Marcello Raspa9, Alessandro Marcello10, Michael P. Myers11, Sergio Crovella3, Paolo Carloni5, Serena Zacchigna1,6 1Cardiovascular Biology, 2Molecular Medicine, 3Biotechnology Development, 10Molecular Virology, and 11Protein Networks Laboratories, International Centre for Genetic Engineering and Biotechnology (ICGEB), Padriciano, 34149, Trieste, Italy 4Institute for Maternal and Child Health, IRCCS "Burlo Garofolo", Trieste, Italy 5Computational Biomedicine Section, Institute of Advanced Simulation IAS-5 and Institute of Neuroscience and Medicine INM-9, Forschungszentrum Jülich GmbH, 52425, Jülich, Germany 6Department of Medical, Surgical and Health Sciences, University of Trieste, 34149 Trieste, Italy 7National Laboratory CIB, Area Science Park Padriciano, Trieste, 34149, Italy 8Department of Life Sciences, University of Trieste, Trieste, 34127, Italy 9Consiglio Nazionale delle Ricerche (IBCN), CNR-Campus International Development (EMMA- INFRAFRONTIER-IMPC), Rome, Italy This PDF file includes: Supplementary Methods Supplementary References Supplementary Figures with legends 1 – 18 Supplementary Tables with legends 1 – 5 Supplementary Movie legends 1, 2 Supplementary Methods Cell culture Primary murine fibroblasts were isolated from skin, lung, kidney and hearts of adult CD1, C57BL/6 or aSMA-RFP/COLL-EGFP mice (1) by mechanical and enzymatic tissue digestion. Briefly, tissue was chopped in small chunks that were digested using a mixture of enzymes (Miltenyi Biotec, 130- 098-305) for 1 hour at 37°C with mechanical dissociation followed by filtration through a 70 µm cell strainer and centrifugation. -

CAS Number Index
2334 CAS Number Index CAS # Page Name CAS # Page Name CAS # Page Name 50-00-0 905 Formaldehyde 56-81-5 967 Glycerol 61-90-5 1135 Leucine 50-02-2 596 Dexamethasone 56-85-9 963 Glutamine 62-44-2 1640 Phenacetin 50-06-6 1654 Phenobarbital 57-00-1 514 Creatine 62-46-4 1166 α-Lipoic acid 50-11-3 1288 Metharbital 57-22-7 2229 Vincristine 62-53-3 131 Aniline 50-12-4 1245 Mephenytoin 57-24-9 1950 Strychnine 62-73-7 626 Dichlorvos 50-23-7 1017 Hydrocortisone 57-27-2 1428 Morphine 63-05-8 127 Androstenedione 50-24-8 1739 Prednisolone 57-41-0 1672 Phenytoin 63-25-2 335 Carbaryl 50-29-3 569 DDT 57-42-1 1239 Meperidine 63-75-2 142 Arecoline 50-33-9 1666 Phenylbutazone 57-43-2 108 Amobarbital 64-04-0 1648 Phenethylamine 50-34-0 1770 Propantheline bromide 57-44-3 191 Barbital 64-13-1 1308 p-Methoxyamphetamine 50-35-1 2054 Thalidomide 57-47-6 1683 Physostigmine 64-17-5 784 Ethanol 50-36-2 497 Cocaine 57-53-4 1249 Meprobamate 64-18-6 909 Formic acid 50-37-3 1197 Lysergic acid diethylamide 57-55-6 1782 Propylene glycol 64-77-7 2104 Tolbutamide 50-44-2 1253 6-Mercaptopurine 57-66-9 1751 Probenecid 64-86-8 506 Colchicine 50-47-5 589 Desipramine 57-74-9 398 Chlordane 65-23-6 1802 Pyridoxine 50-48-6 103 Amitriptyline 57-92-1 1947 Streptomycin 65-29-2 931 Gallamine 50-49-7 1053 Imipramine 57-94-3 2179 Tubocurarine chloride 65-45-2 1888 Salicylamide 50-52-2 2071 Thioridazine 57-96-5 1966 Sulfinpyrazone 65-49-6 98 p-Aminosalicylic acid 50-53-3 426 Chlorpromazine 58-00-4 138 Apomorphine 66-76-2 632 Dicumarol 50-55-5 1841 Reserpine 58-05-9 1136 Leucovorin 66-79-5 -

Pdf 836.43 K
Iranian Journal of Pharmaceutical Research (2014), 13 (4): 1183-1190 Copyright © 2014 by School of Pharmacy Received: April 2013 Shaheed Beheshti University of Medical Sciences and Health Services Accepted: September 2013 Original Article Synthesis of Three Rimantadine Schiff Bases and Their Biological Effects on Serum Albumin Bing-Mi Liua, Ping Mab, Xin Wanga, Yu-Mei Konga, Li-Ping Zhanga and Bin Liua* aCollege of Pharmacy, Liaoning University, Shenyang 110036, P. R. China. bDepartment of Clinical Pharmacology, General Hospital of the Rocket Force, Beijing 100088, P.R.China. Abstract Three new rimantadine Schiff bases (RSBs) were prepared, and then the interaction of RSBs with bovine serum albumin (BSA) was investigated using fluorescence, synchronous fluorescence, UV-vis absorption spectroscopy under physiological conditions. The results showed that the three RSBs effectively quenched the intrinsic fluorescence of BSA via static quenching. Binding constant (Ka), number of binding sites (n), and the binding distance (r) between three RSBs and BSA were calculated by Stern-Volmer equation and Förster’s theory in this study. According to the results of displacement experiments of site probes, it was considered that the binding sites were located in hydrophobic cavities in sub-domains IIA of BSA. What is more, synchronous fluorescence studies indicated that the hydrophobicity around tryptophan residues was increased with the addition of rimantadine-o-vanillin (ROV) and rimantadine- 4-methoxy-salicylaldehyde (RMS), while there was no apparent change with the addition of rimantadine-salicylaldehyde (RS). Keywords: Rimantadine schiff base (RSB); Bovine serum albumin (BSA); Interaction; Fluorescence. Introduction viruses, in particular the influenza A virus (6,7). It has also been reported that rimantadine Schiff bases are a category of compounds hydrochloride has some effects on Parkinson’s containing C=N group. -

Medication Appropriateness Index
Last updated 1/17/2019 Medication Appropriateness Index Patient ID# __________ Evaluator _______________________Date_____________________ Drug Code ____________ Drug___________________________________________________ To assess the appropriateness of the drug, please answer the following questions and circle the applicable rating: 1. Is there an indication for the drug? A________ B_______ C________ Z Indicated Not Indicated DK Comments: 2. Is the medication effective for the A________ B_______ C________ Z condition? Effective Ineffective DK Comments: 3. Is the dosage correct? A________ B_______ C + or C - Z Correct Incorrect DK Comments: 4. Are the directions correct? A_______ B_______ C________ Z Correct Incorrect DK Comments: 5. Are the directions practical? A________ B_______ C________ Z Practical Impractical DK Comments: 6. Are there clinically significant drug- A________ B_______ C________ Z drug interactions? Insignificant Significant DK Comments: 7. Are there clinically significant drug- A________ B_______ C________ Z disease/condition interactions? Insignificant Significant DK Comments: 8. Is there unnecessary duplication with A_______ B_______ C________ Z other drug(s)? Necessary Unnecessary DK Comments: 9. Is the duration of therapy acceptable? A________ B_______ C________ Z Acceptable Not acceptable DK Comments: 10. Is this drug the least expensive A________ B_______ C________ Z alternative compared to others of Least Most DK equal utility? expensive expensive Comments: 1 Last updated 1/17/2019 USE OF THE MEDICATION APPROPRIATENESS INDEX (MAI) For further information/articles using the MAI, address inquiries to Joseph T. Hanlon, PharmD, MS, Department of Medicine (Geriatrics), University of Pittsburgh, Kaufmann Medical Building-Suite 514, 3471 Fifth Ave, Pittsburgh, PA 15213, Email: [email protected] A. General Instructions1 This instrument is intended to assess the appropriateness of medications prescribed by a health care provider and to evaluate patients’ self-medication practices. -

SHANDS July/August 2004 at the University of Florida Drugs & Therapy B ◆ U ◆ L ◆ L ◆ E ◆ T ◆ I ◆ N
Volume 18, Number 7 SHANDS July/August 2004 at the University of Florida Drugs & Therapy B ◆ U ◆ L ◆ L ◆ E ◆ T ◆ I ◆ N FORMULARY UPDATE DRUG INFORMATION FORUM The Pharmacy and Therapeutics NSAIDS + ASA = CONFUSION Committee met June 15, 2004. 3 drugs were added in the Formu- atients are seeing advertisements on platelets and prevents the binding lary and 3 drugs were deleted and P promoting the use of acetamino- of aspirin to platelets.1 This led to the designated not available. phen instead of traditional nonsteroi- recommendation that aspirin should be dal anti-inflammatory drugs (NSAIDs), given before administering ibuprofen. like ibuprofen, when they are taking It also led to observational studies that ◆ ADDED low-dose aspirin for the prevention of suggest that chronic use of ibuprofen cardiovascular events. The premise of (and possibly other NSAIDs) may Extended-Release Divalproex ® these warnings is that traditional decrease the effectiveness of low-dose Sodium (Depakote ER by Abbott NSAIDs could negate the beneficial aspirin.2-3 However, a recently done Pharmaceuticals) cardiovascular effects of low dose case-control study showed that the Rimantadine (Flumadine® by aspirin. The advertisements suggest combination of aspirin and ibuprofen Forest Pharmaceuticals) that acetaminophen is the preferable did not increase the incidence of analgesic because it has fewer drug myocardial infarctions.4 Tiotropium (Spiriva® by interactions. Intermittent use of ibuprofen (and Boehringer Ingelheim/Pfizer) ◆ other NSAIDs) has not been shown to alter the cardiovascular protective ◆ DELETED Is acetaminophen effects of low-dose aspirin. Chronic use Bacitracin + Polymyxin B preferable to traditional of ibuprofen alone (without aspirin) may Topical Powder (Polysporin® NSAIDs in patients taking be cardioprotective compared with Topical Powder by Pfizer)* nothing. -

Women's Health Advisor, Jan 2006
WEILL MEDICAL COLLEGE OF CORNELL UNIVERSITY HELPING WOMEN OVER 50 MAKE INFORMED HEALTH DECISIONSTM January 2006 Volume 10 / Number 1 When Alcohol Becomes Troublesome Alcohol abuse in later life is an unrecognized problem—some women may not even realize they are drinking at unhealthy levels IN THIS ISSUE For some women, older age is a happy, fulfill- them at risk for illnesses and accidents, espe- 2 Frontline ing period—for others, it’s a lonely experi- cially falls,” says Dr. Millman. “For older peo- ence. The loss of a spouse or friends may ple who are at home and lonely, drinking • Aspirin may cut deaths in women with heart disease leave women feeling isolated and depressed. allays those feelings of loneliness; it passes • Exercise can add years to These feelings can intensify if there are phys- the time.” life expectancy ical limitations and family members are scat- “Women may be separated, divorced, or • Omega-3 fats in fish can tered across the country. Some women may widowed. Women who are alone at home reduce the risk of dry eye try to medicate depression or isolation with may have a tendency to drink too much due syndrome • Glucosamine and an accessible and inexpensive drug: alcohol. to lack of social constraints. Or, they have a chrondroitin help knee The National Institute on Alcohol Abuse partner who drinks, and it becomes a shared osteoarthritis pain and Alcoholism (NIAAA) reports that around unhealthy behavior, which may lead to vio- one-third of American women consume lence,” notes Denise Russo, PhD, program 3 Health News alcohol regularly. -

Drugs Affectin the Autonomic Nervous System
Fundamentals of Medical Pharmacology Paterson Public Schools Written by Néstor Collazo, Ph.D. Jonathan Hodges, M.D. Tatiana Mikhaelovsky, M.D. for Health and Related Professions (H.A.R.P.) Academy March 2007 Course Description This fourth year course is designed to give students in the Health and Related Professions (H.A.R.P.) Academy a general and coherent explanation of the science of pharmacology in terms of its basic concepts and principles. Students will learn the properties and interactions between chemical agents (drugs) and living organisms for the rational and safe use of drugs in the control, prevention, and therapy of human disease. The emphasis will be on the fundamental concepts as they apply to the actions of most prototype drugs. In order to exemplify important underlying principles, many of the agents in current use will be singled out for fuller discussion. The course will include the following topics: ¾ The History of Pharmacology ¾ Terminology Used in Pharmacology ¾ Drug Action on Living Organisms ¾ Principles of Pharmacokinetics ¾ Dose-Response Relationships ¾ Time-Response Relationships ¾ Human Variability: Factors that will modify effects of drugs on individuals ¾ Effects of Drugs Attributable to Varying Modes of Administration ¾ Drug Toxicity ¾ Pharmacologic Aspects of Drug Abuse and Drug Dependence Pre-requisites Students must have completed successfully the following courses: Biology, Chemistry, Anatomy and Physiology, Algebra I and II Credits: 5 credits Basic Principles of Drug Action Introduction to Pharmacology a. Basic Mechanisms of Drug Actions b. Dose-response relationships c. Drug absorption d. Biotransformation of Drugs e. Pharmacokinetics f. Factors Affecting Drug Distribution g. Drug Allergy and Pharmacogenetics h.