Elemental Analysis of Organic Species with Electron Ionization High-Resolution Mass Spectrometry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Analytical Techniques Used for Elemental Analysis in Various Matrices
Helaluddin et al Tropical Journal of Pharmaceutical Research February 2016; 15 (2): 427-434 ISSN: 1596-5996 (print); 1596-9827 (electronic) © Pharmacotherapy Group, Faculty of Pharmacy, University of Benin, Benin City, 300001 Nigeria. All rights reserved. Available online at http://www.tjpr.org http://dx.doi.org/10.4314/tjpr.v15i2.29 Review Article Main Analytical Techniques Used for Elemental Analysis in Various Matrices ABM Helaluddin1, Reem Saadi Khalid1, Mohamed Alaama and Syed Atif Abbas2 1Analytical and Bio-Analytical Research Laboratory, Department of Pharmaceutical Chemistry, Faculty of Pharmacy, International Islamic University Malaysia (IIUM), Jalan Istana, Bandar Indera Mahkota, 25200 Kuantan, Pahang, 2 Malaysia School of Pharmacy, Taylors University, 1 Jalan Taylor’s, 47500 Subang Jaya, Selangor Darul Ehsan, Malaysia *For correspondence: Email: [email protected]; [email protected] Received: 20 August 2015 Revised accepted: 4 January 2016 Abstract Heavy metal pollution is a serious environmental problem. The presence of such metals in different areas of an ecosystem subsequently leads to the contamination of consumable products such as dietary and processed materials. Accurate monitoring of metal concentrations in various samples is of importance in order to minimize health hazards resulting from exposure to such toxic substances. For this purpose, it is essential to have a general understanding of the basic principles for different methods of elemental analysis. This article provides an overview of the most sensitive -

Why the Gas Chromatographic Separation Method Used in the Thermo Scientific Flashsmart Elemental Analyzer Is the Most Reliable for Elemental Analysis?
FlashSmart Elemental Analyzer SmartNotes Why the gas chromatographic separation method used in the Thermo Scientific FlashSmart Elemental Analyzer is the most reliable for elemental analysis? The Thermo Scientific™ FlashSmart™ Elemental Analyzer (Figure 1) operates with the dynamic flash combustion (modified Dumas Method) of the sample for CHNS determination while for oxygen analysis, the system operates in pyrolysis mode. The resulted gases are carried by a helium (or argon) flow till a gas chromatographic column that provides the separation of the gases, and finally, detected by a thermal conductivity detector (TCD). A complete report is automatically generated by the Thermo Scientific™ EagerSmart™ Data Handling Software and displayed at the end of the analysis. Thermo Scientific FlashSmart: The Elemental Analyzer Figure 1. Thermo Scientific FlashSmart Elemental Analyzer. The gas chromatography IC (GC) provides a “real” picture The advantages of the separation method are: of the analytical process during combustion (CHNS) and pyrolysis (O). • “Real” peak of each element. • Easy integration of the peaks by the EagerSmart Data GC technique provides you with complete peak Handling Software. separation and sharp peak shapes, which ensure superior precision and higher sensitivity. • The area of the peak corresponds to the total amount of the element. From the chromatogram you can quantify the amount • Proper quantification of the elements. of elements in your sample and recognize what it is happening inside the analyzer anytime. GC separation • Maintenance free, long lifetime GC column operating features provide you with: for years without the need for replacement: it is not a consumable. • Full insight of the combustion showing complete • GC Column easy to use, directly installed in the analyzer conversion of: • Straightforward continuous flow design from sample – Nitrogen and nitrogen oxide in N 2 processing through gas separation and detection. -

Stark Broadening Measurements in Plasmas Produced by Laser Ablation of Hydrogen Containing Compounds Miloš Burger, Jorg Hermann
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Archive Ouverte en Sciences de l'Information et de la Communication Stark broadening measurements in plasmas produced by laser ablation of hydrogen containing compounds Miloš Burger, Jorg Hermann To cite this version: Miloš Burger, Jorg Hermann. Stark broadening measurements in plasmas produced by laser ablation of hydrogen containing compounds. Spectrochimica Acta Part B: Atomic Spectroscopy, Elsevier, 2016, 122, pp.118-126. 10.1016/j.sab.2016.06.005. hal-02348424 HAL Id: hal-02348424 https://hal.archives-ouvertes.fr/hal-02348424 Submitted on 5 Nov 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Stark broadening measurements in plasmas produced by laser ablation of hydrogen containing compounds Milosˇ Burgera,∗,Jorg¨ Hermannb aUniversity of Belgrade, Faculty of Physics, POB 44, 11000 Belgrade, Serbia bLP3, CNRS - Aix-Marseille University, 13008 Marseille, France Abstract We present a method for the measurement of Stark broadening parameters of atomic and ionic spectral lines based on laser ablation of hydrogen containing compounds. Therefore, plume emission spectra, recorded with an echelle spectrometer coupled to a gated detector, were compared to the spectral radiance of a plasma in local thermal equi- librium. -

Good Practice Guide for Isotope Ratio Mass Spectrometry, FIRMS (2011)
Good Practice Guide for Isotope Ratio Mass Spectrometry Good Practice Guide for Isotope Ratio Mass Spectrometry First Edition 2011 Editors Dr Jim Carter, UK Vicki Barwick, UK Contributors Dr Jim Carter, UK Dr Claire Lock, UK Acknowledgements Prof Wolfram Meier-Augenstein, UK This Guide has been produced by Dr Helen Kemp, UK members of the Steering Group of the Forensic Isotope Ratio Mass Dr Sabine Schneiders, Germany Spectrometry (FIRMS) Network. Dr Libby Stern, USA Acknowledgement of an individual does not indicate their agreement with Dr Gerard van der Peijl, Netherlands this Guide in its entirety. Production of this Guide was funded in part by the UK National Measurement System. This publication should be cited as: First edition 2011 J. F. Carter and V. J. Barwick (Eds), Good practice guide for isotope ratio mass spectrometry, FIRMS (2011). ISBN 978-0-948926-31-0 ISBN 978-0-948926-31-0 Copyright © 2011 Copyright of this document is vested in the members of the FIRMS Network. IRMS Guide 1st Ed. 2011 Preface A few decades ago, mass spectrometry (by which I mean organic MS) was considered a “black art”. Its complex and highly expensive instruments were maintained and operated by a few dedicated technicians and its output understood by only a few academics. Despite, or because, of this the data produced were amongst the “gold standard” of analytical science. In recent years a revolution occurred and MS became an affordable, easy to use and routine technique in many laboratories. Although many (rightly) applaud this popularisation, as a consequence the “black art” has been replaced by a “black box”: SAMPLES GO IN → → RESULTS COME OUT The user often has little comprehension of what goes on “under the hood” and, when “things go wrong”, the inexperienced operator can be unaware of why (or even that) the results that come out do not reflect the sample that goes in. -

Elemental Analysis: CHNS/O Determination in Carbon
APPLICATION NOTE 42182 Elemental Analysis: CHNS/O determination in carbon Authors Introduction Dr. Liliana Krotz and Carbon occurs as a variety of allotropes. There are two crystalline forms, Dr. Guido Giazzi diamond and graphite, and a number of amorphous (non-crystalline) forms, Thermo Fisher Scientific, such as charcoal, coke, and carbon black. The most common use of carbon Milan, Italy black is as a pigment and reinforcing phase in automobile tires. Coke is the solid carbonaceous material derived from destructive distillation of low-ash, Keywords low-sulfur bituminous coal. Coke is also used as a fuel and as a reducing Coal, Coke, Carbon Black, agent in smelting iron ore in a blast furnace. Graphite, CHNS/O, Heat Value For quality control purposes, the organic elements in carbon need to be Goal determined. For the determination of carbon, hydrogen, nitrogen, sulfur and This application note reports data oxygen, the combustion method is used. on CHNS/O determination on carbon samples needed for quality The Thermo Scientific™ FlashSmart™ Elemental Analyzer (Figure 1) allows control purposes, performed with the quantitative determination of carbon, hydrogen, nitrogen and oxygen in the FlashSmart EA. carbon. The FlashSmart EA based on the dynamic flash combustion of the sample, provides automated and simultaneous CHNS determination in a single analysis run and oxygen determination by pyrolysis in a second run. To perform total sulfur determination at trace levels, the analyzer has been coupled with the Flame Photometric Detector (FPD). Methods For CHNS determination, the FlashSmart EA operates according to the dynamic flash combustion of the sample. Liquid samples are weighed in tin containers and introduced into the combustion reactor via the Thermo Scientific™ MAS Plus Autosampler. -

Theoretical Calculations for Electron Impact Ionization of Atoms and Molecules
Scholars' Mine Doctoral Dissertations Student Theses and Dissertations Fall 2017 Theoretical calculations for electron impact ionization of atoms and molecules Sadek Mohamed Fituri Amami Follow this and additional works at: https://scholarsmine.mst.edu/doctoral_dissertations Part of the Atomic, Molecular and Optical Physics Commons Department: Physics Recommended Citation Amami, Sadek Mohamed Fituri, "Theoretical calculations for electron impact ionization of atoms and molecules" (2017). Doctoral Dissertations. 2617. https://scholarsmine.mst.edu/doctoral_dissertations/2617 This thesis is brought to you by Scholars' Mine, a service of the Missouri S&T Library and Learning Resources. This work is protected by U. S. Copyright Law. Unauthorized use including reproduction for redistribution requires the permission of the copyright holder. For more information, please contact [email protected]. THEORETICAL CALCULATIONS FOR ELECTRON IMPACT IONIZATION OF ATOMS AND MOLECULES by SADEK MOHAMED FITURI AMAMI A DISSERTATION Presented to the Graduate Faculty of the MISSOURI UNIVERSITY OF SCIENCE AND TECHNOLOGY In Partial Fulfillment of the Requirements for the Degree DOCTOR OF PHILOSOPHY in PHYSICS 2017 Approved by Don H. Madison, Advisor Jerry L. Peacher Michael Schulz Daniel Fischer Gregory Gelles Copyright 2017 SADEK MOHAMED FITURI AMAMI All Rights Reserved iii PUBLICATION DISSERTATION OPTION This dissertation consists of the following ten articles which have been published as follows: Paper I: Pages 31-54 have been published by Phys Rev A.91.032707 (March 2015). Paper II: Pages 55-78 have been published by Phys Rev A.90.012704 (July 2014). Paper III: Pages 79-93 have been published by J. Phys. B: At. Mol. Opt. Phys. 49 185202 (September 2016). -

The Role of Nanoanalytics in the Development of Organic-Inorganic Nanohybrids—Seeing Nanomaterials As They Are
nanomaterials Review The Role of Nanoanalytics in the Development of Organic-Inorganic Nanohybrids—Seeing Nanomaterials as They Are Daria Semenova 1 and Yuliya E. Silina 2,* 1 Process and Systems Engineering Center (PROSYS), Department of Chemical and Biochemical Engineering, Technical University of Denmark, 2800 Kgs. Lyngby, Denmark; [email protected] 2 Institute of Biochemistry, Saarland University, 66123 Saarbrücken, Germany * Correspondence: [email protected] or [email protected]; Tel.: +49-681-302-64717 Received: 23 October 2019; Accepted: 19 November 2019; Published: 23 November 2019 Abstract: The functional properties of organic-inorganic (O-I) hybrids can be easily tuned by combining system components and parameters, making this class of novel nanomaterials a crucial element in various application fields. Unfortunately, the manufacturing of organic-inorganic nanohybrids still suffers from mechanical instability and insufficient synthesis reproducibility. The control of the composition and structure of nanosurfaces themselves is a specific analytical challenge and plays an important role in the future reproducibility of hybrid nanomaterials surface properties and response. Therefore, appropriate and sufficient analytical methodologies and technical guidance for control of their synthesis, characterization and standardization of the final product quality at the nanoscale level should be established. In this review, we summarize and compare the analytical merit of the modern analytical methods, viz. Fourier transform infrared spectroscopy (FTIR), RAMAN spectroscopy, surface plasmon resonance (SPR) and several mass spectrometry (MS)-based techniques, that is, inductively coupled plasma mass spectrometry (ICP-MS), single particle ICP-MS (sp-ICP-MS), laser ablation coupled ICP-MS (LA-ICP-MS), time-of-flight secondary ion mass spectrometry (TOF-SIMS), liquid chromatography mass spectrometry (LC-MS) utilized for characterization of O-I nanohybrids. -

Electron Ionization
Chapter 6 Chapter 6 Electron Ionization I. Introduction ......................................................................................................317 II. Ionization Process............................................................................................317 III. Strategy for Data Interpretation......................................................................321 1. Assumptions 2. The Ionization Process IV. Types of Fragmentation Pathways.................................................................328 1. Sigma-Bond Cleavage 2. Homolytic or Radical-Site-Driven Cleavage 3. Heterolytic or Charge-Site-Driven Cleavage 4. Rearrangements A. Hydrogen-Shift Rearrangements B. Hydride-Shift Rearrangements V. Representative Fragmentations (Spectra) of Classes of Compounds.......... 344 1. Hydrocarbons A. Saturated Hydrocarbons 1) Straight-Chain Hydrocarbons 2) Branched Hydrocarbons 3) Cyclic Hydrocarbons B. Unsaturated C. Aromatic 2. Alkyl Halides 3. Oxygen-Containing Compounds A. Aliphatic Alcohols B. Aliphatic Ethers C. Aromatic Alcohols D. Cyclic Ethers E. Ketones and Aldehydes F. Aliphatic Acids and Esters G. Aromatic Acids and Esters 4. Nitrogen-Containing Compounds A. Aliphatic Amines B. Aromatic Compounds Containing Atoms of Nitrogen C. Heterocyclic Nitrogen-Containing Compounds D. Nitro Compounds E. Concluding Remarks on the Mass Spectra of Nitrogen-Containing Compounds 5. Multiple Heteroatoms or Heteroatoms and a Double Bond 6. Trimethylsilyl Derivative 7. Determining the Location of Double Bonds VI. Library -
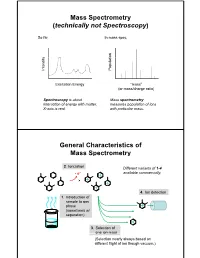
Mass Spectrometry (Technically Not Spectroscopy)
Mass Spectrometry (technically not Spectroscopy) So far, In mass spec, on y Populati Intensit Excitation Energy “mass” (or mass/charge ratio) Spectroscopy is about Mass spectrometry interaction of energy with matter. measures population of ions X-axis is real. with particular mass. General Characteristics of Mass Spectrometry 2. Ionization Different variants of 1-4 -e- available commercially. 4. Ion detection 1. Introduction of sample to gas phase (sometimes w/ separation) 3. Selection of one ion mass (Selection nearly always based on different flight of ion though vacuum.) General Components of a Mass Spectrometer Lots of choices, which can be mixed and matched. direct injection The Mass Spectrum fragment “daughter” ions M+ “parent” mass Sample Introduction: Direc t Inser tion Prob e If sample is a liquid, sample can also be injected directly into ionization region. If sample isn’t pure, get multiple parents (that can’t be distinguished from fragments). Capillary Column Introduction Continous source of molecules to spectrometer. detector column (including GC, LC, chiral, size exclusion) • Signal intensity depends on both amount of molecule and ionization efficiency • To use quantitatively, must calibrate peaks with respect eltilution time ttlitotal ion curren t to quantity eluted (TIC) over time Capillary Column Introduction Easy to interface with gas or liquid chromatography. TIC trace elution time time averaged time averaged mass spectrum mass spectrum Methods of Ionization: Electron Ionization (EI) 1 - + - 1 M + e (kV energy) M + 2e Fragmentation in Electron Ionization daughter ion (observed in spectrum) neutral fragment (not observed) excited parent at electron at electron energy of energy of 15 eV 70 e V Lower electron energy yields less fragmentation, but also less signal. -

An Organic Chemist's Guide to Electrospray Mass Spectrometric
molecules Review An Organic Chemist’s Guide to Electrospray Mass Spectrometric Structure Elucidation Arnold Steckel 1 and Gitta Schlosser 2,* 1 Hevesy György PhD School of Chemistry, ELTE Eötvös Loránd University, Pázmány Péter sétány 1/A, 1117 Budapest, Hungary; [email protected] 2 Department of Analytical Chemistry, ELTE Eötvös Loránd University, Pázmány Péter sétány 1/A, 1117 Budapest, Hungary * Correspondence: [email protected] Received: 16 January 2019; Accepted: 8 February 2019; Published: 10 February 2019 Abstract: Tandem mass spectrometry is an important tool for structure elucidation of natural and synthetic organic products. Fragmentation of odd electron ions (OE+) generated by electron ionization (EI) was extensively studied in the last few decades, however there are only a few systematic reviews available concerning the fragmentation of even-electron ions (EE+/EE−) produced by the currently most common ionization techniques, electrospray ionization (ESI) and atmospheric pressure chemical ionization (APCI). This review summarizes the most important features of tandem mass spectra generated by collision-induced dissociation fragmentation and presents didactic examples for the unexperienced users. Keywords: tandem mass spectrometry; MS/MS fragmentation; collision-induced dissociation; CID; ESI; structure elucidation 1. Introduction Electron ionization (EI), a hard ionization technique, is the method of choice for analyses of small (<1000 Da), nonpolar, volatile compounds. As its name implies, the technique involves ionization by electrons with ~70 eV energy. This energy is high enough to yield very reproducible mass spectra with a large number of fragments. However, these spectra frequently lack the radical type molecular ions (M+) due to the high internal energy transferred to the precursors [1]. -

Methods of Ion Generation
Chem. Rev. 2001, 101, 361−375 361 Methods of Ion Generation Marvin L. Vestal PE Biosystems, Framingham, Massachusetts 01701 Received May 24, 2000 Contents I. Introduction 361 II. Atomic Ions 362 A. Thermal Ionization 362 B. Spark Source 362 C. Plasma Sources 362 D. Glow Discharge 362 E. Inductively Coupled Plasma (ICP) 363 III. Molecular Ions from Volatile Samples. 364 A. Electron Ionization (EI) 364 B. Chemical Ionization (CI) 365 C. Photoionization (PI) 367 D. Field Ionization (FI) 367 IV. Molecular Ions from Nonvolatile Samples 367 Marvin L. Vestal received his B.S. and M.S. degrees, 1958 and 1960, A. Spray Techniques 367 respectively, in Engineering Sciences from Purdue Univesity, Layfayette, IN. In 1975 he received his Ph.D. degree in Chemical Physics from the B. Electrospray 367 University of Utah, Salt Lake City. From 1958 to 1960 he was a Scientist C. Desorption from Surfaces 369 at Johnston Laboratories, Inc., in Layfayette, IN. From 1960 to 1967 he D. High-Energy Particle Impact 369 became Senior Scientist at Johnston Laboratories, Inc., in Baltimore, MD. E. Low-Energy Particle Impact 370 From 1960 to 1962 he was a Graduate Student in the Department of Physics at John Hopkins University. From 1967 to 1970 he was Vice F. Low-Energy Impact with Liquid Surfaces 371 President at Scientific Research Instruments, Corp. in Baltimore, MD. From G. Flow FAB 371 1970 to 1975 he was a Graduate Student and Research Instructor at the H. Laser Ionization−MALDI 371 University of Utah, Salt Lake City. From 1976 to 1981 he became I. -

Elemental and Isotopic Characterization of Tobacco from Umbria
H OH metabolites OH Article Elemental and Isotopic Characterization of Tobacco from Umbria Luana Bontempo 1,*, Daniela Bertoldi 2, Pietro Franceschi 1, Fabio Rossi 3 and Roberto Larcher 2 1 Research and Innovation Centre, Fondazione Edmund Mach (FEM), Via E. Mach 1, 38098 San Michele all’Adige, Italy; [email protected] 2 Technology Transfer Centre, Experiment and Technological Services Department, Fondazione Edmund Mach (FEM), Via E. Mach 1, 38098 San Michele all’Adige, Italy; [email protected] (D.B.); [email protected] (R.L.) 3 Fattoria Autonoma Tabacchi Soc. Coop Agricola, Via G. Oberdan, 06012 Città di Castello, Italy; [email protected] * Correspondence: [email protected]; Tel.: +39-461-615-138 Abstract: Umbrian tobacco of the Virginia Bright variety is one of the most appreciated tobaccos in Europe, and one characterized by an excellent yield. In recent years, the Umbria region and local producers have invested in introducing novel practices (for production and processing) focused on environmental, social, and economic sustainability. Due to this, tobacco from Umbria is a leading commodity in the global tobacco industry, and it claims a high economic value. The aim of this study is then to assess if elemental and isotopic compositions can be used to protect the quality and geographical traceability of this particular tobacco. For the first time the characteristic value ranges of the stable isotope ratios of the bio-elements as a whole (δ2H, δ13C, δ15N, δ18O, and δ34S) and of the concentration of 56 macro- and micro-elements are now available, determined in Virginia Bright tobacco produced in two different areas of Italy (Umbria and Veneto), and from other worldwide Citation: Bontempo, L.; Bertoldi, D.; geographical regions.