WO 2015/127416 Al 27 August 2015 (27.08.2015) P O P C T
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nitrate Prodrugs Able to Release Nitric Oxide in a Controlled and Selective
Europäisches Patentamt *EP001336602A1* (19) European Patent Office Office européen des brevets (11) EP 1 336 602 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.7: C07C 205/00, A61K 31/00 20.08.2003 Bulletin 2003/34 (21) Application number: 02425075.5 (22) Date of filing: 13.02.2002 (84) Designated Contracting States: (71) Applicant: Scaramuzzino, Giovanni AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU 20052 Monza (Milano) (IT) MC NL PT SE TR Designated Extension States: (72) Inventor: Scaramuzzino, Giovanni AL LT LV MK RO SI 20052 Monza (Milano) (IT) (54) Nitrate prodrugs able to release nitric oxide in a controlled and selective way and their use for prevention and treatment of inflammatory, ischemic and proliferative diseases (57) New pharmaceutical compounds of general effects and for this reason they are useful for the prep- formula (I): F-(X)q where q is an integer from 1 to 5, pref- aration of medicines for prevention and treatment of in- erably 1; -F is chosen among drugs described in the text, flammatory, ischemic, degenerative and proliferative -X is chosen among 4 groups -M, -T, -V and -Y as de- diseases of musculoskeletal, tegumental, respiratory, scribed in the text. gastrointestinal, genito-urinary and central nervous sys- The compounds of general formula (I) are nitrate tems. prodrugs which can release nitric oxide in vivo in a con- trolled and selective way and without hypotensive side EP 1 336 602 A1 Printed by Jouve, 75001 PARIS (FR) EP 1 336 602 A1 Description [0001] The present invention relates to new nitrate prodrugs which can release nitric oxide in vivo in a controlled and selective way and without the side effects typical of nitrate vasodilators drugs. -

RAP-MD-05 GLYX13-C-203 Protocol Amendment 3 Final-R
NCT02192099 Study ID: GLYX13‐C‐203 Title: Open Label Extension for Subjects with Inadequate/Partial Response to Antidepressants during the Current Episode of Major Depressive Disorder Previously Treated with Rapastinel (GLYX‐13) (Extension of GLYX13‐C‐202, NCT01684163) Protocol Amendment 3 Date: 23 Nov 2016 Clinical Study Protocol Protocol Number: GLYX13-C-203 Study Drug: Rapastinel (GLYX-13) Injection (rapastinel Injection) FDA ID: 107,974 Clinicaltrials.gov: Title: Open Label Extension for Subjects with Inadequate/Partial Response to Antidepressants during the Current Episode of Major Depressive Disorder Previously Treated with Rapastinel (GLYX-13) (Extension of GLYX13-C-202, NCT01684163) Study Phase: 2 Sponsor: Naurex, Inc, an affiliate of Allergan, plc. Date: Amendment 3 dated 23 November 2016 Replaces Amendment 2 dated 20 January 2015 This document is a confidential communication from Naurex, Inc, an affiliate of Allergan, plc.. Acceptance of this document constitutes an agreement by the recipient(s) that no information contained herein will be published or disclosed without prior written approval from Allergan, plc., except that this document may be disclosed to appropriate Institutional Review Boards (IRBs) under the condition that they are also required to maintain confidentiality. Naurex, Inc, an affiliate of Allergan, plc. Protocol GLYX13-C-203 INVESTIGATOR SIGNATURE PAGE The signature of the investigator below constitutes his/her approval of this protocol and provides the necessary assurances that this study will be conducted according to all stipulations of the protocol as specified in both the clinical and administrative sections, including all statements regarding confidentiality. This trial will be conducted in compliance with the protocol and all applicable regulatory requirements, in accordance with Good Clinical Practices (GCPs), including International Conference on Harmonization (ICH) Guidelines, and in general conformity with the most recent version of the Declaration of Helsinki. -

Anti-Infectives Industry Over the Next 5 Years and Beyond
Bridging the innovation gap... New Drug Futures: Products that could change the pharma market to 2013 and beyond Over 70 pipeline prospects This new major and insightful 450 page in 8 major therapy areas analysis evaluates, compares and contrasts the are analysed in this report prospects for the development compounds that could revolutionise the pharmaceutical Anti-infectives industry over the next 5 years and beyond. Cardiovascular CNS The report provides: Gastrointestinal Detailed background and market context for Metabolic each therapy area covered: Musculoskeletal Addressable patient population Oncology Current treatments Sales drivers Respiratory Sales breakers Future treatments Market dynamics – winners and losers Key drug launches by 2013 Unique sales forecasts by major product to 2013 Over 70 key products assessed Unique evaluation scores for key areas such as novelty of mechanism, clinical data and competition Critical and detailed appraisal of each product‟s research and development Extensive pipeline listings, putting the profiled products into their competitive context The search – and need – for new products has never been greater and what’s in the development pipeline has never generated more interest. That is why this analysis is so important! GLOBAL PHARMA MARKET IN CONTEXT THE Are there too many prophets of doom ready to write-off the research-based pharma industry in the future? Too few novel There is plenty on which to base such anxiety. The research-based industry products and an must achieve a fair price in the face of greater cost control, while the aggressive generic burden of regulation is setting the bar high for successful product sector are taking introduction. -

Targeting Nitric Oxide and NMDA Receptor-Associated Pathways in Treatment of High Grade Glial Tumors
Nitric Oxide 79 (2018) 68–83 Contents lists available at ScienceDirect Nitric Oxide journal homepage: www.elsevier.com/locate/yniox Targeting nitric oxide and NMDA receptor-associated pathways in treatment of high grade glial tumors. Hypotheses for nitro-memantine and nitrones T ∗ Meric A. Altinoz , İlhan Elmaci Neuroacademy Group, Department of Neurosurgery, Memorial Hospital, Istanbul, Turkey ARTICLE INFO ABSTRACT Keywords: Glioblastoma multiforme (GBM) is a devastating brain cancer with no curative treatment. Targeting Nitric Oxide Nitric oxide (NO) and glutamatergic pathways may help as adjunctive treatments in GBM. NO at low doses promotes tu- Nitrone morigenesis, while at higher levels (above 300 nM) triggers apoptosis. Gliomas actively secrete high amounts of Nitro-memantine glutamate which activates EGR signaling and mediates degradation of peritumoral tissues via excitotoxic injury. Glial tumor Memantine inhibits NMDA-subtype of glutamate receptors (NMDARs) and induces autophagic death of glioma Glioblastoma cells in vitro and blocks glioma growth in vivo. Nitro-memantines may exert further benefits by limiting NMDAR signaling and by delivery of NO to the areas of excessive NMDAR activity leading NO-accumulation at tumor- icidal levels within gliomas. Due to the duality of NO in tumorigenesis, agents which attenuate NO levels may also act beneficial in treatment of GBM. Nitrone compounds including N-tert-Butyl-α-phenylnitrone (PBN) and its disulfonyl-phenyl derivative, OKN-007 suppress free radical formation in experimental cerebral ischemia. OKN-007 failed to show clinical efficacy in stroke, but trials demonstrated its high biosafety in humans including elderly subjects. PBN inhibits the signaling pathways of NF-κB, inducible nitric oxide synthase (iNOS) and cy- clooxygenase (COX). -

Poster Session III, December 6, 2017
Neuropsychopharmacology (2017) 42, S476–S652 © 2017 American College of Neuropsychopharmacology. All rights reserved 0893-133X/17 www.neuropsychopharmacology.org Poster Session III a high-resolution research tomograph (HRRT), and struc- Palm Springs, California, December 3–7, 2017 tural (T1) MRI using a 3-Tesla scanner. PET data analyses were carried out using the validated 2-tissue compartment Sponsorship Statement: Publication of this supplement is model to determine the total volume of distribution (VT) of sponsored by the ACNP. [18F]FEPPA. Individual contributor disclosures may be found within the Results: Results show significant inverse associations (con- abstracts. Part 1: All Financial Involvement with a pharma- trolling for rs6971 genotype) between neuroinflammation ceutical or biotechnology company, a company providing (VTs) and cortical thickness in the right medial prefrontal clinical assessment, scientific, or medical products or compa- cortex [r = -.562, p = .029] and the left dorsolateral nies doing business with or proposing to do business with prefrontal cortex [r = -.629, p = .012](p values are not ACNP over past 2 years (Calendar Years 2014–Present); Part 2: corrected for multiple comparisons). No significant associa- Income Sources & Equity of $10,000 per year or greater tions were found with surface area. (Calendar Years 2014 - Present): List those financial relation- Conclusions: These results, while preliminary, suggest links ships which are listed in part one and have a value greater than between the microglial activation/neuroinflammation and $10,000 per year, OR financial holdings that are listed in part cortical thickness in the dorsolateral prefrontal cortex and one and have a value of $10,000 or greater as of the date of medial prefrontal cortex in AD patients. -

Stems for Nonproprietary Drug Names
USAN STEM LIST STEM DEFINITION EXAMPLES -abine (see -arabine, -citabine) -ac anti-inflammatory agents (acetic acid derivatives) bromfenac dexpemedolac -acetam (see -racetam) -adol or analgesics (mixed opiate receptor agonists/ tazadolene -adol- antagonists) spiradolene levonantradol -adox antibacterials (quinoline dioxide derivatives) carbadox -afenone antiarrhythmics (propafenone derivatives) alprafenone diprafenonex -afil PDE5 inhibitors tadalafil -aj- antiarrhythmics (ajmaline derivatives) lorajmine -aldrate antacid aluminum salts magaldrate -algron alpha1 - and alpha2 - adrenoreceptor agonists dabuzalgron -alol combined alpha and beta blockers labetalol medroxalol -amidis antimyloidotics tafamidis -amivir (see -vir) -ampa ionotropic non-NMDA glutamate receptors (AMPA and/or KA receptors) subgroup: -ampanel antagonists becampanel -ampator modulators forampator -anib angiogenesis inhibitors pegaptanib cediranib 1 subgroup: -siranib siRNA bevasiranib -andr- androgens nandrolone -anserin serotonin 5-HT2 receptor antagonists altanserin tropanserin adatanserin -antel anthelmintics (undefined group) carbantel subgroup: -quantel 2-deoxoparaherquamide A derivatives derquantel -antrone antineoplastics; anthraquinone derivatives pixantrone -apsel P-selectin antagonists torapsel -arabine antineoplastics (arabinofuranosyl derivatives) fazarabine fludarabine aril-, -aril, -aril- antiviral (arildone derivatives) pleconaril arildone fosarilate -arit antirheumatics (lobenzarit type) lobenzarit clobuzarit -arol anticoagulants (dicumarol type) dicumarol -

Intravesical Cocktails PBS/IC
International Painful Bladder Foundation Interstitial Cystitis/Painful Bladder Syndrome Anaesthetic intravesical cocktails 1. Anaesthetic cocktail – Robert Moldwin, MD 1:1 mixture of 0.5% Marcaine and 2% Lidocaine jelly – about 40 cc total. To this solution are added: Heparin sulphate 10,000 IU Triamcinolone 40 mg Gentamycin 80 mg or a post-procedural prophylactic antibiotic. Administration: Patients are instructed to hold the solution for about 30 minutes, then to void. When given as a diagnostic test, patients will generally sense relief of pain within 5-10 minutes. The only (rare) problems that we’ve encountered are the following: Patients may experience “rebound” pain once the solution has worn off (within 3-5 hours). This generally resolves with continued instillations. When given as therapy, we usually administer the cocktail on a weekly basis for 8-12 weeks. This is the length of time usually needed to get a prolonged response. Then, the duration between instillations is increased to q 2 weeks to q 3 weeks, etc., ultimately with the goal of discontinuance. Patients may experience urinary retention requiring catheterization. This seems to be particularly a problem in patients who appear to have pre-existing voiding dysfunction, those patients who initially present with a poor urinary flow rate, an interrupted urinary stream, etc. The urinary retention can usually be circumvented by delivering a lower total volume. 2. Marcaine with steroid cocktail – Nagendra Mishra, MD Marcaine 40 ml Heparin sulphate 10,000 IU Dexamethasone 2 cc Sodium bicarbonate 20 ml Administration: This cocktail should be held in the bladder for 20 minutes. It should be administered every 15 days for a total of 6 treatments and then as needed. -
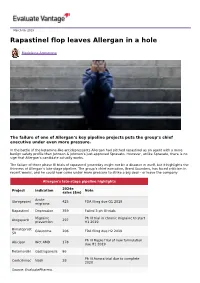
Rapastinel Flop Leaves Allergan in a Hole
March 06, 2019 Rapastinel flop leaves Allergan in a hole Madeleine Armstrong The failure of one of Allergan’s key pipeline projects puts the group’s chief executive under even more pressure. In the battle of the ketamine-like antidepressants Allergan had pitched rapastinel as an agent with a more benign safety profile than Johnson & Johnson’s just-approved Spravato. However, unlike Spravato, there is no sign that Allergan’s candidate actually works. The failure of three phase III trials of rapastinel yesterday might not be a disaster in itself, but it highlights the thinness of Allergan’s late-stage pipeline. The group’s chief executive, Brent Saunders, has faced criticism in recent weeks, and he could now come under more pressure to strike a big deal – or leave the company. Allergan's late-stage pipeline highlights 2024e Project Indication Note sales ($m) Acute Ubrogepant 425 FDA filing due Q1 2019 migraine Rapastinel Depression 359 Failed 3 ph III trials Migraine Ph III trial in chronic migraine to start Atogepant 297 prevention H1 2019 Bimatoprost Glaucoma 206 FDA filing due H2 2019 SR Ph III Maple trial of new formulation Abicipar Wet AMD 178 due H1 2019 Relamorelin Gastroparesis 86 Ph III Aurora trial due to complete Cenicriviroc Nash 28 2020 Source: EvaluatePharma. Activist shareholders have long been agitating for change at Allergan, and the latest failure will only provide more ammunition to those who want to split the roles of chairman and chief exec amid dissatisfaction with the group's acquisition strategy, and with its stock hovering around a five-year low. -

WO 2017/066590 Al 20 April 2017 (20.04.2017) P O P CT
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2017/066590 Al 20 April 2017 (20.04.2017) P O P CT (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 38/06 (2006.01) A61K 31/519 (2006.01) kind of national protection available): AE, AG, AL, AM, A61 38/07 (2006.0V) A61K 31/551 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, A61K 31/13 (2006.01) A61K 31/5513 (2006.01) BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, A61K 31/135 (2006.01) A61P 25/24 (2006.01) DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, A61K 31/496 (2006.01) A61P 25/18 (2006.01) HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, (21) International Application Number: MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, PCT/US20 16/057071 OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, (22) International Filing Date: SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, 14 October 2016 (14.10.201 6) TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, zw. (25) Filing Language: English (84) Designated States (unless otherwise indicated, for every (26) Publication Language: English kind of regional protection available): ARIPO (BW, GH, (30) Priority Data: GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, 62/242,633 16 October 2015 (16. -

Evaluation of Pentosan Polysulfate Sodium in the Postoperative Recovery from Cranial Cruciate Injury in Dogs: a Randomized
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/6367210 Evaluation of Pentosan Polysulfate Sodium in the Postoperative Recovery from Cranial Cruciate Injury in Dogs: A Randomized... Article in Veterinary Surgery · May 2007 DOI: 10.1111/j.1532-950X.2007.00256.x · Source: PubMed CITATIONS READS 16 103 4 authors, including: Steven C Budsberg Mary Sarah Bergh University of Georgia Iowa State University 129 PUBLICATIONS 3,106 CITATIONS 22 PUBLICATIONS 366 CITATIONS SEE PROFILE SEE PROFILE All content following this page was uploaded by Steven C Budsberg on 11 March 2014. The user has requested enhancement of the downloaded file. Veterinary Surgery 36:234–244, 2007 Evaluation of Pentosan Polysulfate Sodium in the Postoperative Recovery from Cranial Cruciate Injury in Dogs: A Randomized, Placebo-Controlled Clinical Trial STEVEN C. BUDSBERG, DVM, MS, Diplomate ACVS, MARY SARAH BERGH, DVM, LISA R. REYNOLDS, BS, and HEATHER K. STREPPA, DVM, Diplomate ACVS Objective—To evaluate the efficacy of pentosan polysulfate (PPS) for improving the recovery period and mitigate the progression of osteoarthritis (OA) of the canine stifle after extracapsular stabilization of cranial cruciate ligament (CCL) injuries. Study Design—Randomized, blinded, placebo-controlled clinical trial. Animals—Dogs (n ¼ 40) with unilateral CCL instability. Methods—Each dog had an extracapsular stabilization of the stifle with or without partial men- iscectomy. Dogs were divided into 4 groups based on preoperative radiographic assessment and whether a partial meniscectomy was performed. Dogs were randomly assigned to either (3 mg/kg) PPS or placebo treatment in each group, and then injected subcutaneously weekly for 4 weeks. -
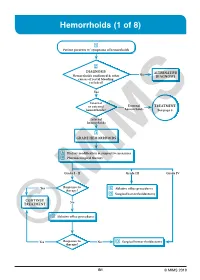
Hemorrhoids (1 of 8)
Hemorrhoids (1 of 8) 1 Patient presents w/ symptoms of hemorrhoids 2 DIAGNOSIS No ALTERNATIVE Hemorrhoids confi rmed & other DIAGNOSIS causes of rectal bleeding excluded? Yes Internal or external External TREATMENT hemorrhoids? hemorrhoids See page 3 Internal hemorrhoids 3 GRADE HEMORRHOIDS A Dietary modifi cation & supportive measures B Pharmacological therapy Grade I - II Grade III Grade IV Yes Response to C Ablative offi ce procedures therapy? D Surgical hemorrhoidectomy CONTINUE No TREATMENT C Ablative offi ce procedures Yes Response to No D Surgical hemorrhoidectomy ©therapy? MIMS B1 © MIMS 2019 Hemorrhoids (2 of 8) 1 SYMPTOMS ATTRIBUTED TO HEMORRHOIDS • Rectal bleeding - Most common presenting symptom - Bright red blood which may drip or squirt into the toilet bowl or scanty amounts may be seen on toilet tissue • Discomfort due to rectal protrusion or lump • Anal pain • HEMORRHOIDS Anal itching 2 DIAGNOSIS Medical History • Assess nature, duration & severity of symptoms - Ask about bleeding, its amount & frequency - Ask about presence of prolapsing tissue, its timing & reproducibility • Elicit possible risk factors for development of hemorrhoidal symptoms - Low-fi ber diets cause small-caliber stools, resulting in straining during defecation & engorgement of hemorrhoids - Prolonged sitting on a toilet which may cause a problem in the venous return in the perianal area - Pregnancy - Advanced age • Th e signs & symptoms of hemorrhoids are not specifi c to the disease, so care must be taken to avoid missing other causes of pathology • Obtain -

Management of Joint Disease in the Sport Horse
1 MANAGEMENT OF JOINT DISEASE IN THE SPORT HORSE Management of Joint Disease in the Sport Horse C. WAYNE MCILWRAITH Colorado State University, Ft. Collins, Colorado INTRODUCTION The joint is an organ, and there are a number of ways in which traumatic damage occurs, ultimately resulting in degradation of articular cartilage. It was recognized in 1966 that articular cartilage change that accompanied osteochondral fragmentation could also be associated with concurrent traumatic damage to the attachment of the joint capsule and ligaments (Raker et al., 1966). However, there was little association made between primary disease in the synovial membrane and fibrous joint capsule and the development of osteoarthritic change in the articular cartilage until an experimental study demonstrated that cartilage degradation could occur in the horse in the absence of instability or trau- matic disruption of tissue and that loss of glycosaminoglycan (GAG) staining was associated with early morphologic breakdown at the surface of the cartilage (McIlwraith and Van Sickle, 1984). Surveys have confirmed that approximately 60% of lameness problems are related to osteoarthritis (National Animal Health Monitoring Systems, 2000; Caron and Genovese, 2003). Rapid resolution of synovitis and capsuli- tis is a critical part of the medical treatment of joint disease because of the principal role of synovitis in causing cartilage matrix breakdown. The goal of treatment of traumatic entities of the joint is twofold: (1) returning the joint to normal as quickly as possible, and (2) preventing the occurrence or reduction of the severity of osteoarthritis. In other words, treatment is intended to (1) reduce pain (lameness), and (2) minimize progression of joint deterioration.