O Showed That Decarboxylation of Lauroyloxy Radicals Are O-( + Too Fast to Be Trapped by MSD and the Free-Radical >-O "
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) United States Patent (10) Patent No.: US 7.449,439 B2 to Et Al
USOO7449439B2 (12) United States Patent (10) Patent No.: US 7.449,439 B2 to et al. (45) Date of Patent: Nov. 11, 2008 (54) WATER-SOLUBLE THICKENER AND LIQUID (56) References Cited ACDIC DETERGENT U.S. PATENT DOCUMENTS (75) Inventors: Kenji Ito, Aichi (JP); Yoshio Mori, 3,768,565 A 10, 1973 Persinski et al. Aichi (JP) (Continued) (73) Assignee: Toagosei Co., Ltd., Tokyo (JP) FOREIGN PATENT DOCUMENTS (*) Notice: Subject to any disclaimer, the term of this JP 53-463O2 A 4f1978 patent is extended or adjusted under 35 U.S.C. 154(b) by 455 days. (Continued) (21) Appl. No.: 10/530,179 OTHER PUBLICATIONS International Search Report PCT/JP2003/012764 dated Dec. 9, 2003. (22) PCT Filed: Oct. 6, 2003 (Continued) (86). PCT No.: PCT/UP03/12764 Primary Examiner Brian PMruk S371 (c)(1), (74) Attorney, Agent, or Firm Sughrue Mion, PLLC (2), (4) Date: Apr. 4, 2005 (57) ABSTRACT (87) PCT Pub. No.: WO2004/031314 A water-soluble thickener which is highly effective even in PCT Pub. Date: Apr. 15, 2004 thickening strongly acidic aqueous Solutions and has excel lent stability in such solutions. It comprises a water-soluble (65) Prior Publication Data copolymer having a weight-average molecular weight of 6,000,000 or higher obtainable by polymerizing a monomer US 2006/OO46949 A1 Mar. 2, 2006 mixture which comprises 2-acrylamido-2-methylpropane (30) Foreign Application Priority Data Sulfonic acid and/or a salt thereofandacrylic acid and/or a salt thereofas essential components and optionally one or more Oct. 4, 2002 (JP) ............................. 2002-292975 other copolymerizable monomer components including the Oct. -

Hydroxylamines and Hydrazines As Surrogates of Sp3 Carbons in Medicinal Chemistry
Wayne State University Wayne State University Dissertations January 2018 Hydroxylamines And Hydrazines As Surrogates Of Sp3 Carbons In Medicinal Chemistry Sandeep Dhanju Wayne State University, [email protected] Follow this and additional works at: https://digitalcommons.wayne.edu/oa_dissertations Part of the Chemistry Commons Recommended Citation Dhanju, Sandeep, "Hydroxylamines And Hydrazines As Surrogates Of Sp3 Carbons In Medicinal Chemistry" (2018). Wayne State University Dissertations. 2156. https://digitalcommons.wayne.edu/oa_dissertations/2156 This Open Access Dissertation is brought to you for free and open access by DigitalCommons@WayneState. It has been accepted for inclusion in Wayne State University Dissertations by an authorized administrator of DigitalCommons@WayneState. HYDROXYLAMINES AND HYDRAZINES AS SURROGATES OF SP3 CARBONS IN MEDICINAL CHEMISTRY by SANDEEP DHANJU DISSERTATION Submitted to the Graduate School of Wayne State University, Detroit, Michigan in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY 2019 MAJOR: CHEMISTRY (Organic) Approved By: Advisor Date DEDICATION I dedicate my PhD work to my parents Sanubhai Shrestha and Punamaya Dhanju, for their endless love and support. ii ACKNOWLEDGEMENTS First and foremost, I would like to express my sincere gratitude to my Ph.D. advisor Prof. David Crich for his incessant support and guidance for my Ph.D. research work during the past five years in his laboratory. I am grateful and fortunate to be one of his graduate students. His encouragement and motivation drove me to the end of this thesis, and I was able to develop an in-depth knowledge and enthusiasm in the areas of organic chemistry and medicinal chemistry. I would like to extend my gratitude to Prof. -

Chemical Hygiene Plan Ii Revised 03/2021 Table of Contents
MAR 2021 Office of Environmental Health and Safety Principal Author/Editor: David Webber, PhD/Chemical Hygiene Officer Contributing Authors/Editors: Nikolai Evdokimov, PhD, James Gibson, PhD, Tania Guardado, PhD, Amanda Jevons, Deona Willes, MPH Graphics/Design: Alfred M. Bouziane, MS, Brent Pantell USC Chemical Hygiene Plan ii Revised 03/2021 Table of Contents i.0 2021 Revision Summary Section 3.0 vi Section 4.0 vi Section 5.0 vii Section 7.0 vii Section 8.0 viii Section 10.0 x Appendix D x Appendix G x 1.0 Introduction Purpose and Scope 1.1 Sources of Safety Information 1.2 2.0 Regulatory Requirements 3.0 Roles and Responsibilities Research Safety Oversight Committee (RSOC) 3.1 Campus-Wide Chemical Safety Committee (CCSC) 3.1 Other Safety Committees 3.2 Office of Environmental Health & Safety 3.2 Principal Investigator (PI) 3.3 Training Requirements 3.5 4.0 Basics of Laboratory Safety Hazard, Risk, and Safety Management 4.1 Hierarchy of Safety Controls 4.1 Group Safety Management and Safety Culture 4.4 USC Chemical Hygiene Plan iii Revised 03/2021 Basics of Lab Facilities, Equipment, and Emergency Supplies 4.7 Emergency Equipment and Supplies 4.15 Open Flames 4.20 5.0 Hazard Communication Labeling and Signage Systems 5.2 Labelling and Signage in the Lab: What You Need to Do 5.5 Safety Data Sheets (SDSs): What Are They? 5.7 SDSs in The Lab: What You Need to Do 5.8 6.0 Hazardous Chemicals and Hazard Classification Introduction 6.1 Health-Hazardous Chemicals: Routes of Exposure 6.2 Particularly Hazardous Substances (PHS) 6.16 7.0 Chemical -

United States Patent Office 2,918,478 Patented Dec
United States Patent Office 2,918,478 Patented Dec. 22, 1959 2 and non-protonic acids, the vinylene carbonate is poly 2,918,478 merized. The polymerizing agents referred to include: WNYLENE CARBONATE AND METHODS OF Benzoyl peroxide PREPARING T Actyl peroxide Melvin S. Newman, Upper Arlington, Ohio, assignor to Di-t-butyl peroxide corporationThe Ohio Stateof Ohio University Research Foundation, a GN GN Dimethyl azobisisobutyronitrile- CH-i-N-N-(-CH, No Drawing. Original application June 1, 1954, Serial CE CH3 No. 433,795. Divided and this application April 16, O Sulfuric acid 1957, Serial No. 653,053 Hydrochloric acid 6 Claims. (CI. 260-340.2) Hydrofluoric acid Perchloric acid This application is the sole application of Melvin S. Aluminum chloride Newman and is a division of a co-pending joint applica s Zinc chloride tion of Melvin S. Newman and Roger W. Addor, Serial Boron trifluoride No. 433,795, filed June 1, 1954, which itself is in part Hydrogen chloride-aluminum chloride a continuation of copending application Serial No. Hydrogen fluoride-boron trifluoride 338,654, filed February 25, 1953. The invention disclosed in this application relates to 20 On treating with a nitrating agent, an aminating agent, vinylene carbonate; to homo polymers thereof; and to an acetylating agent, an alkylating agent (e.g. an ether derivatives of such vinylene carbonate and of such poly fying agent), a carboxymethylating agent, or with other mers and to processes for the syntheses of such com agents, I react the vinylene carbonate, the polymers, or pounds. the derivatives of such polymers further. One of the objects is therefore the preparation of 25 PREPARATION OF VENYLENE CARBONATE vinylene carbonate. -

Polymer Synthesis and Characterization
Basic Laboratory Course For Polymer Science M. Sc. Program POLYMER SYNTHESIS AND CHARACTERIZATION WS 2011/12 Department of Chemistry and Biochemistry Free University of Berlin Edited by Florian Paulus, Dirk Steinhilber, Tobias Becherer Polymer Synthesis and Characterization – Basic Laboratory Course Table of Contents 1. Experiments .......................................................................................................... 1 1.1. Free Radical Chain Growth Polymerization .......................................................... 1 1.1.1. Bulk Polymerization of MMA with AIBN (Trommsdorff-Norrish Effect) ............... 1 1.1.2. Suspension Polymerization of Styrene with DBPO ............................................... 3 1.1.3. Emulsion Polymerization of Styrene ..................................................................... 4 1.1.4. Polymer Analogues Reaction: Synthesis and Characterization of Sodium Carboxymethylcellulose ........................................................................................ 5 1.1.5. Free Radical Copolymerization of MMA and Styrene ........................................... 6 1.1.6. Precipitation Polymerization of Acrylonitrile with Redox Initiator in Water........ 7 1.1.7. Photo polymerization of PEG-400-diacrylate ....................................................... 9 1.1.8. Dilatometry ......................................................................................................... 10 1.2. Anionic Polymerization ...................................................................................... -

Direct Cyanation, Hydrocyanation, Dicyanation And
RSC Advances REVIEW View Article Online View Journal | View Issue Direct cyanation, hydrocyanation, dicyanation and cyanofunctionalization of alkynes Cite this: RSC Adv., 2020, 10,10232 Lifen Peng, *a Zhifang Hu,a Hong Wang,a Li Wu,a Yinchun Jiao,a Zilong Tanga and Xinhua Xu *b In this review, direct cyanation, hydrocyanation, dicyanation, cyanofunctionalization and other cyanation reactions of alkynes were highlighted. Firstly, the use of nitriles and development of cyanation was simply introduced. After presenting the natural properties of alkynes, cyanation reactions of alkynes were classified and introduced in detail. Transition metal catalysed direct cyanation and hydrocyanation of Received 10th February 2020 alkynes gave alkynyl cyanides and alkenyl nitriles in good yields. Dicyanation of alkynes produced 1,2- Accepted 4th March 2020 dicyano adducts. Cyanofunctionalization of alkynes afforded functional cyanated compounds. DOI: 10.1039/d0ra01286f Thiocyanation and selenocyanation yielded the expected functional vinylthiocyanates and rsc.li/rsc-advances vinylselenocyanates. A plausible reaction mechanism is presented if available. Creative Commons Attribution-NonCommercial 3.0 Unported Licence. Introduction benzyl cyanide,10 acetonitrile,11 N,N-dimethylformamide (DMF),12 azobisisobutyronitrile (AIBN),13 trimethylsilylcyanide (TMSCN),14 15 The nitrile group, an equivalent to carbonyl, carboxyl, amino and ary(cyano)iodonium triates, N-cyano-N-phenyl-p-toluenesulfo- 16 17 hydroxymethyl groups, has been considered as not only a key namide -

Copolymer Comprising Units of Type a Deriving from Carboxylic Acid Monomers and Units of Type B Deriving from Sulfonic Acid Monomers
(19) TZZ ¥_T (11) EP 2 896 637 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 22.07.2015 Bulletin 2015/30 C08F 293/00 (2006.01) C11D 3/37 (2006.01) (21) Application number: 14305084.7 (22) Date of filing: 21.01.2014 (84) Designated Contracting States: • Wilson, David James AL AT BE BG CH CY CZ DE DK EE ES FI FR GB 60580 Coye la Foret (FR) GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO • Clavier, Julie PL PT RO RS SE SI SK SM TR 92600 Asnieres sur Seine (FR) Designated Extension States: • van Gramberen, Eric BA ME 95870 Bezons (FR) (71) Applicant: Rhodia Operations (74) Representative: Nony 93306 Aubervilliers Cedex (FR) 3, rue de Penthièvre 75008 Paris (FR) (72) Inventors: •Orizet,Céline 92340 Bourg la Reine (FR) (54) Copolymer comprising units of type A deriving from carboxylic acid monomers and units of type B deriving from sulfonic acid monomers (57) The present invention relates to a copolymer of type A and B with a molar ratio of units of type A to comprising units of type A deriving from carboxylic acid units of type B greater or equal to 1, wherein said units monomers and units of type B deriving from sulfonic acid of type A and units of type B are statistically distributed, monomers, said units of type A and B representing more said second block having a degree of polymerization DP 2 than 80 mol % of the total moles of units in the copolymer, such that DP2/DP1 ≥ 1. -
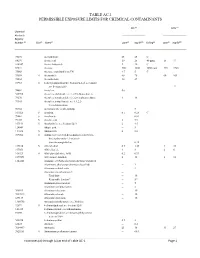
Table Ac-1 Permissible Exposure Limits for Chemical Contaminants
TABLE AC-1 PERMISSIBLE EXPOSURE LIMITS FOR CHEMICAL CONTAMINANTS PEL (d) STEL (o) Chemical Abstracts Registry Number (a) (b) (c) (e) 3(f) (g) (e) 3(f) Skin Name ppm mg/M Ceiling ppm mg/M 75070 Acetaldehyde 25 45 C 64197 Acetic acid 10 25 40 ppm 15 37 108247 Acetic Anhydride 5 20 C 67641 Acetone 500 1200 3000 ppm 750 1780 75868 Acetone cyanohydrin as CN 4.7 5 C 75058 S Acetonitrile 40 70 60 105 98862 Acetophenone 10 49 53963 S 2-Acetylaminofluorene; N-fluoren-2-yl acetamide; see Section 5209 12 74862 Acetylene (h) 540590 Acetylene dichloride; see 1,2-Dichloroethylene 79276 Acetylene tetrabromide:1,1,2,2-tetrabromoethane 1 14 79345 Acetylene tetrachloride; see 1,1,2,2- Tetrachloroethane 50782 Acetylsalicylic acid (Aspirin) 5 107028 S Acrolein 0.1 0.25 C 79061 S Acrylamide -- 0.03 79107 S Acrylic acid 2 5.9 107131 S Acrylonitrile; see Section 5213 2 4.5 124049 Adipic acid -- 5 111693 S Adiponitrile 2 8.8 309002 S Aldrin; 1,2,3,4,10,10-hexachloro-1,4,4a,5,8,8a- hexahydro-endo-1,2-exo-5,8- dimethanonaphthalene -- 0.25 107186 S Allyl alcohol 0.5 1.25 4 10 107051 Allyl chloride 1 3 2 6 106923 S Allyl glycidyl ether; AGE 0.2 0.93 2179591 Allyl propyl disulfide 2 12 3 18 1344281 Alumina; see Particulates not otherwise regulated Aluminum, alkyls (not otherwise classified) -- 2 Aluminum soluble salts -- 2 Aluminum metal and oxide -- Total dust -- 10 Respirable fraction(n) -- 5(n) Aluminum pyro powders -- 5 Aluminum welding fumes -- 5 300925 Aluminum distearate -- 10 7047849 Aluminum stearate -- 10 637127 Aluminum tristearate -- 10 1300738 Aminodimethylbenzene; -

Propargylation of Nitroalkanes Via The
PROPARGYLATION OF NITROALKANES VIA THE COPPER-CATALYZED CROSS COUPLING by Linh Dinh-Nguyen A thesis submitted to the Faculty of the University of Delaware in partial fulfillment of the requirements for the degree of Master of Science in Chemistry and Biochemistry Fall 2019 © 2019 Linh Dinh-Nguyen All Rights Reserved PROPARGYLATION OF NITROALKANES VIA THE COPPER–CATALYZED CROSS COUPLING by Linh Dinh-Nguyen Approved: _____________________________________________________ Donald A. Watson, Ph.D. Professor in charge of thesis on behalf of the Advisory Committee Approved: _____________________________________________________ Brian J. Bahnson, Ph.D. Chair of the Department of Chemistry and Biochemistry Approved: _____________________________________________________ John A. Pelesko, Ph.D. Dean of the College of Arts and Sciences Approved: _____________________________________________________ Douglas J. Doren, Ph.D. Interim Vice Provost for Graduate and Professional Education Dean of the Graduate College ACKNOWLEDGMENTS Most importantly, I would like to express my gratitude to my advisor, Professor Donald Watson, for his mentorship and support during my time at the University of Delaware. Thank you for welcoming me into your group and giving me critical scientific suggestions and advice. Your knowledge and deep passion to chemistry has inspired me to become a better scientist and individual. I would like to express my gratitude to all dedicated faculty and staffs at the UDel Chemistry and Biochemistry department. I would like to thank all the professors for teaching the most informative and challenging graduate courses I have ever taken. Especially, Professor Mary Watson and Professor Joel Rosenthal’s courses and scientific suggestions have made a great impact in my research journey. Also, I would like to thank Dr. -

Chemical Hygiene Plan
CHEMICAL HYGIENE PLAN Developed by HSU Environmental Health & Safety in partial fulfillment of the California Code of Regulations Title 8, §5191 Occupational Exposure to Hazardous Chemicals in Laboratories Effective 7/1/12 Humboldt State University Chemical Hygiene Plan I. REGULATORY AUTHORITY II. ADMINISTERING AGENCY III. POLICY IV. PURPOSE V. SCOPE VI. RESPONSIBILITIES, TITLE 8 CCR, §5919 (e)(3)(G) VII. STANDARD OPERATING PROCEDURES, TITLE 8 CCR, §5919 (e)(3)(A) VIII. CONTROL MEASURES AND EQUIPMENT, TITLE 8 CCR, §5919 (e)(3)(B) IX. MEDICAL CONSULTATION AND MONITORING, TITLE 8 CCR, §5919 (e)(3)(F) X. PARTICULARLY HAZARDOUS SUBSTANCES, TITLE 8 CCR, §5919 (e)(3)(H) XI. TRAINING, TITLE 8 CCR, §5919 (e)(3)(D) XII. APPROVAL PROCEDURES, TITLE 8 CCR, §5919 (e)(3)(E) XIII. PROCUREMENT AND GIFTS XIV. UPSET CONDITIONS/SPILLS, RELEASES AND ACCIDENTS XV. HAZARDOUS WASTE MANAGEMENT XVI. RECORDS AND RECORDKEEPING XVII. CHANGES TO THE UNIVERSITY CHEMICAL HYGIENE PLAN XVIII. DEFINITIONS APPENDIX A: Select Carcinogens APPENDIX B: Cal/OSHA Permissible Exposure Limits for Chemical Contaminants APPENDIX C: Title 8 CCR, §5191 – Occupational Exposure to Chemicals in Laboratories APPENDIX D: Chemical Compatibility Chart APPENDIX E: Peroxide-Forming Chemicals APPENDIX F: Laboratory Inspection Checklist APPENDIX G: PPE Selection Guide APPENDIX H: Select Agents and Toxins List I. REGULATORY AUTHORITY a. U.S. authority is codified in the Code of Federal Regulations, Title 29, §1910.1450 b. California State authority is codified in the California Code of Regulations, Title 8, §5191 (see APPENDIX C) i. Unless otherwise indicated, California regulations shall supersede Federal regulations. II. ADMINISTERING AGENCY a. The administering agency for occupational health and safety in California is the Department of Industrial Relations, Occupational Health and Safety Administration (Cal/OSHA) i. -

Isocyanate-Free Routes to Polyurethanes and Poly(Hydroxy Urethane)S Lise Maisonneuve, Oceane Lamarzelle, Estelle Rix, Etienne Grau, Henri Cramail
Isocyanate-Free Routes to Polyurethanes and Poly(hydroxy Urethane)s Lise Maisonneuve, Oceane Lamarzelle, Estelle Rix, Etienne Grau, Henri Cramail To cite this version: Lise Maisonneuve, Oceane Lamarzelle, Estelle Rix, Etienne Grau, Henri Cramail. Isocyanate-Free Routes to Polyurethanes and Poly(hydroxy Urethane)s. Chemical Reviews, American Chemical Soci- ety, 2015, 115 (22), pp.12407-12439. 10.1021/acs.chemrev.5b00355. hal-01365096 HAL Id: hal-01365096 https://hal.archives-ouvertes.fr/hal-01365096 Submitted on 26 Nov 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Isocyanate-free routes to polyurethanes and poly(hydroxy urethane)s Lise Maisonneuve,1,2 Océane Lamarzelle,1,2 Estelle Rix, 1,2 Etienne Grau1,2 and Henri Cramail1,2* 1 Univ. Bordeaux, LCPO, UMR 5629, F-33600, Pessac, France ; 2 CNRS, LCPO, UMR 5629, F-33600, Pessac, France *[email protected] 1 Abstract __________________________________________________________________ 4 Introduction _______________________________________________________________ 4 1- Transurethanization: towards phosgene-free -

United States Patent 19 11 Patent Number: 5,698,647 Huckestein Et Al
US005698647A United States Patent 19 11 Patent Number: 5,698,647 Huckestein et al. 45 Date of Patent: Dec. 16, 1997 2,927,102 3/1960 Wolfgang et al. ................... 526/219.5 54 PREPARATION OF POLYMERS BASED ON BASC VNYL MONOMERS 3,006,900 10/1961 Fikentscher et al. ................ 526/219.5 4,701,494 10/1987 Graafiland ............................. 526,219.5 75 Inventors: Brigitta Huckestein, Schifferstadt; FOREIGN PATENT DOCUMENTS Wolker Schehlmann, Römerberg; Axel Sanner, Frankenthal; Rainer 559630 1/1980 Japan .................................. 526/219.5 Blankenburg, Ludwigshafen, all of 188006 10/1966 U.S.S.R. ............................. 526/219.5 Germany WO93/16114 8/1993 WIPO : OTHER PUBLICATIONS (73) Assignee: BASF Aktiengesellschaft, Ludwigshafen, Germany Derwent Publ, Abst.JP 57 092 422. Chem. Abst, vol. 87, No. 22, 1977, Abs. No. 168624b 21 Appl. No.: 697,724 (English abstract of JP 52 071586). Primary Examiner-Joseph L. Schofer 22 Filed: Aug. 29, 1996 Assistant Examiner-N. Sarofim 30 Foreign Application Priority Data Attorney Agent, or Firm-Keil & Weinkauf Sep. 8, 1996 DEI Germany ........................ 19533 217.2 57 ABSTRACT (51) int. C. m. CO8F 2/16 A process for the preparation of low-hydrazine polymers 52 U.S. Cl. ............ 526/219.5; 526/264 based on basic vinyl monomers by free-radical solution 58) Field of Search ....................................... 526/219.5 polymerization in aqueous medium in the presence, as compound which forms free radicals, of an azo compound 56) References Cited which has alkoxycarbonyl groups on the carbon atoms U.S. PATENT DOCUMENTS adjacent to the azo group. 2,643,990 6/1953 Ham .................................... 526.219.5 2 Claims, No Drawings 5,698,647 1.