STUDIES on the DISSOCIATION of MULLITE Thesis Submitted for the Degree of Doctor of Philosophy in the University of London Naimu
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

New Minerals Approved Bythe Ima Commission on New
NEW MINERALS APPROVED BY THE IMA COMMISSION ON NEW MINERALS AND MINERAL NAMES ALLABOGDANITE, (Fe,Ni)l Allabogdanite, a mineral dimorphous with barringerite, was discovered in the Onello iron meteorite (Ni-rich ataxite) found in 1997 in the alluvium of the Bol'shoy Dolguchan River, a tributary of the Onello River, Aldan River basin, South Yakutia (Republic of Sakha- Yakutia), Russia. The mineral occurs as light straw-yellow, with strong metallic luster, lamellar crystals up to 0.0 I x 0.1 x 0.4 rnrn, typically twinned, in plessite. Associated minerals are nickel phosphide, schreibersite, awaruite and graphite (Britvin e.a., 2002b). Name: in honour of Alia Nikolaevna BOG DAN OVA (1947-2004), Russian crys- tallographer, for her contribution to the study of new minerals; Geological Institute of Kola Science Center of Russian Academy of Sciences, Apatity. fMA No.: 2000-038. TS: PU 1/18632. ALLOCHALCOSELITE, Cu+Cu~+PbOZ(Se03)P5 Allochalcoselite was found in the fumarole products of the Second cinder cone, Northern Breakthrought of the Tolbachik Main Fracture Eruption (1975-1976), Tolbachik Volcano, Kamchatka, Russia. It occurs as transparent dark brown pris- matic crystals up to 0.1 mm long. Associated minerals are cotunnite, sofiite, ilin- skite, georgbokiite and burn site (Vergasova e.a., 2005). Name: for the chemical composition: presence of selenium and different oxidation states of copper, from the Greek aA.Ao~(different) and xaAxo~ (copper). fMA No.: 2004-025. TS: no reliable information. ALSAKHAROVITE-Zn, NaSrKZn(Ti,Nb)JSi401ZJz(0,OH)4·7HzO photo 1 Labuntsovite group Alsakharovite-Zn was discovered in the Pegmatite #45, Lepkhe-Nel'm MI. -

4. EUDIALYTE-EUCOLITES and PROBLEMS on TYPOMORPHISM of MINERALS Boris Ye
NDM44_eng_09-11_091228:NDM44_02_pek_FMM_0909011.qxd 29.12.2009 18:27 Страница 97 New Data on Minerals. M., 2009. Vol. 44 97 THE ESSAYS ON FUNDAMENTAL AND GENETIC MINERALOGY: 4. EUDIALYTE-EUCOLITES AND PROBLEMS ON TYPOMORPHISM OF MINERALS Boris Ye. Borutzky Institute of Ore Deposit Geology, Petrography, Mineralogy and Geochemistry RAS, Moscow, [email protected] The present paper is a sequel to the previously published (Borutzky, 2008) essay on the minerals of variable com- position with variable structure (MVCVS) with an example of eudialyte-eucolites. The characteristic feature of this typical mineral from alkaline complexes is the unique ability to include up to one third of The Mendeleev’s Periodic Table of the chemical elements in its composition. This is entailed by a partial realignment of the crystal structure in response to changing chemistry and evolution of the mineral-forming environment with time. According to the author, the detailed typomorphous analysis of eudialyte-eucolites is more informative and use- ful in terms of genetic mineralogy rather than formal determination of dozens of independent mineral species. 3 tables, 48 references Keywords: eudialyte-eucolites, minerals of variable composition with variable structure. What is typomorphism and its issues natural chemical compounds, but worked towards determination of the relationship «We have to distinguish two types of between composition, structure, mineral prospecting features: one is related to the properties and conditions of formation, i.e. its nature of the objects sought for (minerals and genetic features in order to use them as the elements) that result from ion and lattice struc- mineralogical indicators for solving problems ture features. -

The Standardisation of Mineral Group Hierarchies: Application to Recent Nomenclature Proposals
Eur. J. Mineral. 2009, 21, 1073–1080 Published online October 2009 The standardisation of mineral group hierarchies: application to recent nomenclature proposals Stuart J. MILLS1,*, Fred´ eric´ HATERT2,Ernest H. NICKEL3,** and Giovanni FERRARIS4 1 Department of Earth and Ocean Sciences, University of British Columbia, Vancouver, BC, V6T 1Z4, Canada Commission on New Minerals, Nomenclature and Classification, of the International Mineralogical Association (IMA–CNMNC), Secretary *Corresponding author, e-mail: [email protected] 2 Laboratoire de Minéralogie et de Cristallochimie, B-18, Université de Liège, 4000 Liège, Belgium IMA–CNMNC, Vice-Chairman 3 CSIRO, Private Bag 5, Wembley, Western Australia 6913, Australia 4 Dipartimento di Scienze Mineralogiche e Petrologiche, Università di Torino, Via Valperga Caluso 35, 10125, Torino, Italy Abstract: A simplified definition of a mineral group is given on the basis of structural and compositional aspects. Then a hier- archical scheme for group nomenclature and mineral classification is introduced and applied to recent nomenclature proposals. A new procedure has been put in place in order to facilitate the future proposal and naming of new mineral groups within the IMA–CNMNC framework. Key-words: mineral group, supergroup, nomenclature, mineral classification, IMA–CNMNC. Introduction History There are many ways which are in current use to help with From time to time, the issue of how the names of groups the classification of minerals, such as: Dana’s New Miner- have been applied and its consistency has been discussed alogy (Gaines et al., 1997), the Strunz classification (Strunz by both the CNMMN/CNMNC and the Commission on & Nickel, 2001), A Systematic Classification of Minerals Classification of Minerals (CCM)1. -

New Data on the Isomorphism in Eudialyte-Group Minerals. 2
minerals Review New Data on the Isomorphism in Eudialyte-Group Minerals. 2. Crystal-Chemical Mechanisms of Blocky Isomorphism at the Key Sites Ramiza K. Rastsvetaeva 1 and Nikita V. Chukanov 2,3,* 1 Shubnikov Institute of Crystallography of Federal Scientific Research Centre “Crystallography and Photonics”, Russian Academy of Sciences, Leninskiy Prospekt 59, 119333 Moscow, Russia; [email protected] 2 Institute of Problems of Chemical Physics, Russian Academy of Sciences, Chernogolovka, 142432 Moscow, Russia 3 Faculty of Geology, Moscow State University, Vorobievy Gory, 119991 Moscow, Russia * Correspondence: [email protected] Received: 24 July 2020; Accepted: 14 August 2020; Published: 17 August 2020 Abstract: The review considers various complex mechanisms of isomorphism in the eudialyte-group minerals, involving both key positions of the heteropolyhedral framework and extra-framework components. In most cases, so-called blocky isomorphism is realized when one group of atoms and ions is replaced by another one, which is accompanied by a change in the valence state and/or coordination numbers of cations. The uniqueness of these minerals lies in the fact that they exhibit ability to blocky isomorphism at several sites of high-force-strength cations belonging to the framework and at numerous sites of extra-framework cations and anions. Keywords: eudialyte group; crystal chemistry; blocky isomorphism; peralkaline rocks 1. Introduction Eudialyte-group minerals (EGMs) are typical components of some kinds of agpaitic igneous rocks and related pegmatites and metasomatic assemblages. Crystal-chemical features of these minerals are important indicators reflecting conditions of their formation (pressure, temperature, fugacity of oxygen and volatile species, and activity of non-coherent elements [1–9]). -

IMA–CNMNC Approved Mineral Symbols
Mineralogical Magazine (2021), 85, 291–320 doi:10.1180/mgm.2021.43 Article IMA–CNMNC approved mineral symbols Laurence N. Warr* Institute of Geography and Geology, University of Greifswald, 17487 Greifswald, Germany Abstract Several text symbol lists for common rock-forming minerals have been published over the last 40 years, but no internationally agreed standard has yet been established. This contribution presents the first International Mineralogical Association (IMA) Commission on New Minerals, Nomenclature and Classification (CNMNC) approved collection of 5744 mineral name abbreviations by combining four methods of nomenclature based on the Kretz symbol approach. The collection incorporates 991 previously defined abbreviations for mineral groups and species and presents a further 4753 new symbols that cover all currently listed IMA minerals. Adopting IMA– CNMNC approved symbols is considered a necessary step in standardising abbreviations by employing a system compatible with that used for symbolising the chemical elements. Keywords: nomenclature, mineral names, symbols, abbreviations, groups, species, elements, IMA, CNMNC (Received 28 November 2020; accepted 14 May 2021; Accepted Manuscript published online: 18 May 2021; Associate Editor: Anthony R Kampf) Introduction used collection proposed by Whitney and Evans (2010). Despite the availability of recommended abbreviations for the commonly Using text symbols for abbreviating the scientific names of the studied mineral species, to date < 18% of mineral names recog- chemical elements -
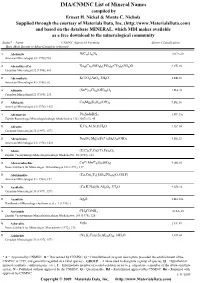
IMA/CNMNC List of Mineral Names
IMA/CNMNC List of Mineral Name s compiled by Ernest H. Nickel & Monte C. Nichols Supplied through the courtesy of Materials Data, Inc. (http://www.MaterialsData.com) and based on the database MINERAL, which MDI makes available as a free download to the mineralogical community Status* Name CNMNC Approved Formula Strunz Classification Best, Most Recent or Most Complete reference. A Abelsonite NiC£¡H£¢N¤ 10.CA.20 American Mineralogist 63 (1978) 930 A Abenakiite-(Ce) Na¢¦Ce¦(SiO£)¦(PO¤)¦(CO£)¦(SO¢)O 9.CK.10 Canadian Mineralogist 32 (1994), 843 G Abernathyite K(UO¢)AsO¤•3H¢O 8.EB.15 American Mineralogist 41 (1956), 82 A Abhurite (SnÀÈ)¢¡Cl¡¦(OH)¡¤O¦ 3.DA.30 Canadian Mineralogist 23 (1985), 233 D Abkhazite Ca¢Mg¥Si¨O¢¢(OH)¢ 9.DE.10 American Mineralogist 63 (1978), 1023 A Abramovite Pb¢SnInBiS§ 2.HF.25a Zapiski Rossiiskogo Mineralogicheskogo Obshchetstva 136 (2007) (5), 45 D Abrazite K,Ca,Al,Si,O,H¢O 9.GC.05 Canadian Mineralogist 35 (1997), 1571 D Abriachanite Na¢(Fe,Mg)£(FeÁÈ)¢Si¨O¢¢(OH)¢ 9.DE.25 American Mineralogist 63 (1978), 1023 D Absite (U,Ca,Y,Ce)(Ti,Fe)¢O¦ Zapiski Vsesoyuznogo Mineralogicheskogo Obshchestva 92 (1963), 113 A Abswurmbachite CuÀÈ(MnÁÈ)¦O¨(SiO¤) 9.AG.05 Neues Jahrbuch für Mineralogie, Abhandlungen 163 (1991), 117 D Abukumalite (Ca,Ce)¢Y£(SiO¤,PO¤)£(O,OH,F) American Mineralogist 51 (1966), 152 D Acadialite (Ca,K,Na)(Si,Al)£O¦•3H¢O 9.GD.10 Canadian Mineralogist 35 (1997), 1571 G Acanthite Ag¢S 2.BA.30a Handbook of Mineralogy (Anthony et al.), 1 (1990), 1 A Acetamide CH£CONH¢ 10.AA.20 Zapiski Vsesoyuznogo Mineralogicheskogo -

Eudialyte Group Minerals from the Lovozero Alkaline Massif, Russia: Occurrence, Chemical Composition, and Petrogenetic Significance
minerals Article Eudialyte Group Minerals from the Lovozero Alkaline Massif, Russia: Occurrence, Chemical Composition, and Petrogenetic Significance Julia A. Mikhailova *, Yakov A. Pakhomovsky, Taras L. Panikorovskii, Ayya V. Bazai and Victor N. Yakovenchuk Kola Science Centre, Russian Academy of Sciences, 14 Fersman Street, 184209 Apatity, Russia; [email protected] (Y.A.P.); [email protected] (T.L.P.); [email protected] (A.V.B.); [email protected] (V.N.Y.) * Correspondence: [email protected]; Tel.: +7-81555-79333 Received: 4 October 2020; Accepted: 27 November 2020; Published: 29 November 2020 Abstract: The Lovozero Alkaline Massif intruded through the Archean granite-gneiss and Devonian volcaniclastic rocks ca. 360 Ma ago and formed a large laccolith-type body. The lower part of the massif (the Layered complex) is composed of regularly repeating rhythms: melanocratic nepheline syenite (lujavrite, at the top), leucocratic nepheline syenite (foyaite), foidolite (urtite). The upper part of the massif (the Eudialyte complex) is indistinctly layered, and lujavrite enriched with eudialyte-group minerals (EGM) prevails there. In this article, we present the results of a study of the chemical composition and petrography of more than 400 samples of the EGM from the main types of rock of the Lovozero massif. In all types of rock, the EGM form at the late magmatic stage later than alkaline clinopyroxenes and amphiboles or simultaneously with it. When the crystallization of pyroxenes and EGM is simultaneous, the content of ferrous iron in the EGM composition increases. The Mn/Fe ratio in the EGM increases during fractional crystallization from lujavrite to foyaite and urtite. -

Pseudomorphs, Trickster Mineral Specimens
Bulletin of the Mineralogical Society of Southern California Volume 93 Number 8 - August, 2020 The 980th meeting of the Mineralogical Society of Southern California With Knowledge Comes Appreciation A ZOOM Meeting August 14th, 2020 at 7:30 P.M. Program: Pseudomorphs, Trickster Mineral Specimens Presented by Howard Heitner In this Issue: TITLE Page Program: Pseudomorphs, Trickster Mineral Specimens Presented by Howard Heitner 2 From the Editor: Linda Elsnau 2 From the President; Interesting Minerals, A to Z. Round 2, installment 5, the letter “E”: Eudialyte by George Rossman 2 Minutes of the July 10, 2020 ZOOM Meeting 4 List of Upcoming MSSC Events 7 Special ZOOM Notice by Rudy Lopez 7 Recent Deaths in the Southern CA Mineral Family 8 Ride Share Listing 8 Other Free Things To Do...by Ann Meister 8 Calendar of Events 9 August Featured Mineral: Wavellite 10 2020 Officers 11 About MSSC 11 Remember: If you change your email or street address, you must let the MSSC Editor and Membership Chair know or we cannot guarantee receipt of future Bulletins About the Program: Pseudomorphs, Trickster Mineral Specimens Presented by Howard Heitner The word pseudomorph was first used by Hauy in 1801. In Greek it means “false shape” A pseudomorph is a mineral that has the external crystal shape of another mineral. The subject of pseudomorphs was first written about in detail by the 19th century German mineralogist Johann Blum. Pseudomorphs were later classified by the mineralogist Strunz in 1982. The talk covers the various theories of how pseudomorphs form. In some cases the second mineral is similar in composition to the original mineral. -

Íîâûå Ìèíåðàëû
2007 ÇÀÏÈÑÊÈ ÐÎÑÑÈÉÑÊÎÃÎ ÌÈÍÅÐÀËÎÃÈ×ÅÑÊÎÃÎ ÎÁÙÅÑÒÂÀ ×. CXXXVI, ¹ 4 2007 ZAPISKI RMO (PROCEEDINGS OFTHE RUSSIAN MINERALOGICAL SOCIETY) Pt CXXXVI, N 4 ÍÎÂÛÅ ÌÈÍÅÐÀËÛ ÓÄÊ 549.6 © Ïî÷åòíûé ÷ë. À. Ï. ÕÎÌßÊÎÂ,* ä. ÷ë. Ã. Í. ÍÅ×ÅËÞÑÒÎÂ,** ä. ÷ë. Ð. Ê. ÐÀÑÖÂÅÒÀÅÂÀ*** ÄÓÀËÈÒ Na30(Ca,Na,Ce,Sr)12(Na,Mn,Fe,Ti)6Zr3Ti3MnSi51O144(OH,H2O,Cl)9 — ÍÎÂÛÉ ÖÈÐÊÎÍÎ-ÒÈÒÀÍÎÑÈËÈÊÀÒ Ñ ÌÎÄÓËßÐÍÎÉ ÝÂÄÈÀËÈÒÎÏÎÄÎÁÍÎÉ ÑÒÐÓÊÒÓÐÎÉ ÈÇ ËÎÂÎÇÅÐÑÊÎÃÎ ÙÅËÎ×ÍÎÃÎ ÌÀÑÑÈÂÀ, ÊÎËÜÑÊÈÉ ÏÎËÓÎÑÒÐÎÂ, ÐÎÑÑÈß1 A. P. KHOMYAKOV, G. N. NECHELYUSTOV, R. K. RASTSVETAEVA. DUALITE, Na30(Ca,Na,Ce,Sr)12(Na,Mn,Fe,Ti)6Zr3Ti3MnSi51O144(OH,H2O,Cl)9, A NEW ZIRCONO-TITANOSILICATE WITH A MODULAR EUDIALYTE-LIKE STRUCTURE FROM LOVOZERO ALKALINE MASSIF, KOLA PENINSULA, RUSSIA * Èíñòèòóò ìèíåðàëîãèè, ãåîõèìèè è êðèñòàëëîõèìèè ðåäêèõ ýëåìåíòîâ, 121357, Ìîñêâà, óë. Âåðåñàåâà, 15; e-mail: [email protected] ** Âñåðîññèéñêèé íàó÷íî-èññëåäîâàòåëüñêèé èíñòèòóò ìèíåðàëüíîãî ñûðüÿ, 109017, Ìîñêâà, Ñòàðîìîíåòíûé ïåð., 31 *** Èíñòèòóò êðèñòàëëîãðàôèè ÐÀÍ, 117333, Ìîñêâà, Ëåíèíñêèé ïð., 59 Dualite was found on Mt. Alluaiv (Lovozero massif, Kola Peninsula), in hyperagpaitic pegmatoid rock as rare anhedral grains up to 0.3—0.5 mm across. Associated minerals are: sodium-potassium feldspar, nepheline, sodali- te, cancrinite, aegirine, alkaline amphibole, eudialyte, lovozerite, lomonosovite, vuonnemite, lamprophyllite, spha- lerite and villiaumite. It is yellow, transparent or translucent, with conchoidal fracture; brittle; luster vitreous; streak white. Mohs’ hardness 5. D(meas.) = 2.84(3) g/cm3 (volumetric method), D(calc.) = 2.814 g/cm3. The mineral dis- solves and gelatinises in acid at room temperature. Non-fluorescent. Optically uniaxial, positive, no = 1.610(1), 3 ne = 1.613(1). Trigonal, space group R3m, a = 14.153(9), c = 60.72(5) Å, V = 10533(22) Å , Z = 3. -

The Essays on Fundamental and Genetic Mineralogy: 3
148 New Data on Minerals. М., 2008. Volume 43 THE ESSAYS ON FUNDAMENTAL AND GENETIC MINERALOGY: 3. MINERALS OF VARIABLE COMPOSITION WITH VARIABLE STRUCTURE AND PROBLEMS OF SPECIES FORMATION IN MINERALOGY. EUDIALYTE-EUCOLITES Boris Ye. Borutzky Institute of Ore Deposit, Geology, Petrography, Mineralogy and Geochemistry, RAS, Moscow, [email protected] This paper is one of a series on genetic and fundamental mineralogy (Borutzky, 2005; 2006) where the problem of mineral species definition are discussed. In particular, the correlation of such fundamental terms in mineralogy as mineral – mineral species – mineral variety are considered, and compared to analogous nomenclature units in biology – another natural science. Also, the necessity for a genetic basis in mineral species definition is shown and the natural genetic taxonomy elaborated and applied to the feldspar group is demonstrated; this approach was used for the corresponding part in “Minerals” reference book (2003). The author insists that formalised structural and chemical criteria for mineral species recommended by the International Mineralogical Association (IMA) Commission on New Minerals, Nomenclature and Classification (CNMNC) without consideration of genesis dis- credits mineralogical science, because the result in unwarranted “reproduction” of abstract species which do not correspond to real discrete mineral entities widespread in certain geological environments. The paper is devoted to the detailed consideration of so-called minerals of variable composition and variable structure defined by the author (Borutzky, 1997; 1999) using the example of eudialyte-eucolites, and also the scientific and opportunistic problems accompanying the study of these chemically and structurally unique minerals. 6 figures, 111 references. Keywords: minerals of variable composition and variable structure, eudialytes-eucolites, new mineral species, the new mineral species criteria.