Flora.Sa.Gov.Au/Jabg
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pima County Plant List (2020) Common Name Exotic? Source
Pima County Plant List (2020) Common Name Exotic? Source McLaughlin, S. (1992); Van Abies concolor var. concolor White fir Devender, T. R. (2005) McLaughlin, S. (1992); Van Abies lasiocarpa var. arizonica Corkbark fir Devender, T. R. (2005) Abronia villosa Hariy sand verbena McLaughlin, S. (1992) McLaughlin, S. (1992); Van Abutilon abutiloides Shrubby Indian mallow Devender, T. R. (2005) Abutilon berlandieri Berlandier Indian mallow McLaughlin, S. (1992) Abutilon incanum Indian mallow McLaughlin, S. (1992) McLaughlin, S. (1992); Van Abutilon malacum Yellow Indian mallow Devender, T. R. (2005) Abutilon mollicomum Sonoran Indian mallow McLaughlin, S. (1992) Abutilon palmeri Palmer Indian mallow McLaughlin, S. (1992) Abutilon parishii Pima Indian mallow McLaughlin, S. (1992) McLaughlin, S. (1992); UA Abutilon parvulum Dwarf Indian mallow Herbarium; ASU Vascular Plant Herbarium Abutilon pringlei McLaughlin, S. (1992) McLaughlin, S. (1992); UA Abutilon reventum Yellow flower Indian mallow Herbarium; ASU Vascular Plant Herbarium McLaughlin, S. (1992); Van Acacia angustissima Whiteball acacia Devender, T. R. (2005); DBGH McLaughlin, S. (1992); Van Acacia constricta Whitethorn acacia Devender, T. R. (2005) McLaughlin, S. (1992); Van Acacia greggii Catclaw acacia Devender, T. R. (2005) Acacia millefolia Santa Rita acacia McLaughlin, S. (1992) McLaughlin, S. (1992); Van Acacia neovernicosa Chihuahuan whitethorn acacia Devender, T. R. (2005) McLaughlin, S. (1992); UA Acalypha lindheimeri Shrubby copperleaf Herbarium Acalypha neomexicana New Mexico copperleaf McLaughlin, S. (1992); DBGH Acalypha ostryaefolia McLaughlin, S. (1992) Acalypha pringlei McLaughlin, S. (1992) Acamptopappus McLaughlin, S. (1992); UA Rayless goldenhead sphaerocephalus Herbarium Acer glabrum Douglas maple McLaughlin, S. (1992); DBGH Acer grandidentatum Sugar maple McLaughlin, S. (1992); DBGH Acer negundo Ashleaf maple McLaughlin, S. -
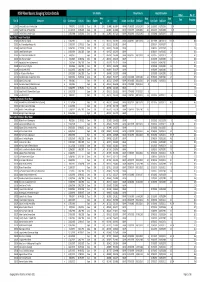
Gauging Station Index
Site Details Flow/Volume Height/Elevation NSW River Basins: Gauging Station Details Other No. of Area Data Data Site ID Sitename Cat Commence Ceased Status Owner Lat Long Datum Start Date End Date Start Date End Date Data Gaugings (km2) (Years) (Years) 1102001 Homestead Creek at Fowlers Gap C 7/08/1972 31/05/2003 Closed DWR 19.9 -31.0848 141.6974 GDA94 07/08/1972 16/12/1995 23.4 01/01/1972 01/01/1996 24 Rn 1102002 Frieslich Creek at Frieslich Dam C 21/10/1976 31/05/2003 Closed DWR 8 -31.0660 141.6690 GDA94 19/03/1977 31/05/2003 26.2 01/01/1977 01/01/2004 27 Rn 1102003 Fowlers Creek at Fowlers Gap C 13/05/1980 31/05/2003 Closed DWR 384 -31.0856 141.7131 GDA94 28/02/1992 07/12/1992 0.8 01/05/1980 01/01/1993 12.7 Basin 201: Tweed River Basin 201001 Oxley River at Eungella A 21/05/1947 Open DWR 213 -28.3537 153.2931 GDA94 03/03/1957 08/11/2010 53.7 30/12/1899 08/11/2010 110.9 Rn 388 201002 Rous River at Boat Harbour No.1 C 27/05/1947 31/07/1957 Closed DWR 124 -28.3151 153.3511 GDA94 01/05/1947 01/04/1957 9.9 48 201003 Tweed River at Braeside C 20/08/1951 31/12/1968 Closed DWR 298 -28.3960 153.3369 GDA94 01/08/1951 01/01/1969 17.4 126 201004 Tweed River at Kunghur C 14/05/1954 2/06/1982 Closed DWR 49 -28.4702 153.2547 GDA94 01/08/1954 01/07/1982 27.9 196 201005 Rous River at Boat Harbour No.3 A 3/04/1957 Open DWR 111 -28.3096 153.3360 GDA94 03/04/1957 08/11/2010 53.6 01/01/1957 01/01/2010 53 261 201006 Oxley River at Tyalgum C 5/05/1969 12/08/1982 Closed DWR 153 -28.3526 153.2245 GDA94 01/06/1969 01/09/1982 13.3 108 201007 Hopping Dick Creek -

Annual Report 2001-2002 (PDF
2001 2002 Annual report NSW national Parks & Wildlife service Published by NSW National Parks and Wildlife Service PO Box 1967, Hurstville 2220 Copyright © National Parks and Wildlife Service 2002 ISSN 0158-0965 Coordinator: Christine Sultana Editor: Catherine Munro Design and layout: Harley & Jones design Printed by: Agency Printing Front cover photos (from top left): Sturt National Park (G Robertson/NPWS); Bouddi National Park (J Winter/NPWS); Banksias, Gibraltar Range National Park Copies of this report are available from the National Parks Centre, (P Green/NPWS); Launch of Backyard Buddies program (NPWS); Pacific black duck 102 George St, The Rocks, Sydney, phone 1300 361 967; or (P Green); Beyers Cottage, Hill End Historic Site (G Ashley/NPWS). NPWS Mail Order, PO Box 1967, Hurstville 2220, phone: 9585 6533. Back cover photos (from left): Python tree, Gossia bidwillii (P Green); Repatriation of Aboriginal remains, La Perouse (C Bento/Australian Museum); This report can also be downloaded from the NPWS website: Rainforest, Nightcap National Park (P Green/NPWS); Northern banjo frog (J Little). www.npws.nsw.gov.au Inside front cover: Sturt National Park (G Robertson/NPWS). Annual report 2001-2002 NPWS mission G Robertson/NPWS NSW national Parks & Wildlife service 2 Contents Director-General’s foreword 6 3Conservation management 43 Working with Aboriginal communities 44 Overview Joint management of national parks 44 Mission statement 8 Aboriginal heritage 46 Role and functions 8 Outside the reserve system 47 Customers, partners and stakeholders -

Indigenous Plants of Bendigo
Produced by Indigenous Plants of Bendigo Indigenous Plants of Bendigo PMS 1807 RED PMS 432 GREY PMS 142 GOLD A Gardener’s Guide to Growing and Protecting Local Plants 3rd Edition 9 © Copyright City of Greater Bendigo and Bendigo Native Plant Group Inc. This work is Copyright. Apart from any use permitted under the Copyright Act 1968, no part may be reproduced by any process without prior written permission from the City of Greater Bendigo. First Published 2004 Second Edition 2007 Third Edition 2013 Printed by Bendigo Modern Press: www.bmp.com.au This book is also available on the City of Greater Bendigo website: www.bendigo.vic.gov.au Printed on 100% recycled paper. Disclaimer “The information contained in this publication is of a general nature only. This publication is not intended to provide a definitive analysis, or discussion, on each issue canvassed. While the Committee/Council believes the information contained herein is correct, it does not accept any liability whatsoever/howsoever arising from reliance on this publication. Therefore, readers should make their own enquiries, and conduct their own investigations, concerning every issue canvassed herein.” Front cover - Clockwise from centre top: Bendigo Wax-flower (Pam Sheean), Hoary Sunray (Marilyn Sprague), Red Ironbark (Pam Sheean), Green Mallee (Anthony Sheean), Whirrakee Wattle (Anthony Sheean). Table of contents Acknowledgements ...............................................2 Foreword..........................................................3 Introduction.......................................................4 -

National Parks Association of the Australian Capital Territory Inc
Volume 53 Number 2 June 2016 National Parks Association of the Australian Capital Territory Inc. Burning Aranda Bushland Canberra Nature Map Jagungal Wilderness NPA Bulletin Volume 53 number 2 June 2016 Articles by contributors may not necessarily reflect association opinion or objectives. CONTENTS NPA outings program, June – September 2016 ...............13–16 From the Committee ................................................................2 Bushwalks Rod Griffiths and Christine Goonrey Exciting Rendezvous Valley pack walk ..........................17 The vital work of the National Parks Australia Council ..........3 Esther Gallant Rod Griffiths Mount Tantangara ...........................................................18 NPA's Nature Play program .....................................................3 Brian Slee Graham Scully Pretty Plain ......................................................................19 Aranda Bushland's recent hazard-reduction burn ....................4 Brian Slee Judy Kelly, with Michael Doherty and John Brickhill Glenburn Precinct news..........................................................20 Obituaries .................................................................................6 Col McAlister Book reviews. Leaf Litter, exploring the Mysteries................21 The National Rock Garden ......................................................7 of a Hidden World by Rachel Tonkin Compiled by Kevin McCue Judy Kelly Stolen .......................................................................................7 -

Association of Societies for Growing Australian Plants
ISSN - 335X November. 1994 ASSOCIATION OF SOCIETIES FOR GROWING AUSTRALIAN PLANTS THE AUSTRALIAN DAISY-STUDY GROUP-NEWSLETTER N0.40 Dear Members, With the bulk of collecting and observation completed for the Brachyscome Project, it looks as if drought will thwart me. Any rain that falls in the next couple of weeks will be too late for my collecting schedule. There's also the irritating problem of some arid area species failing to appreciate the urgency of our situation. They are being reluctant to germinate or to grow on down here in Melbourne. All this suggests there will be plenty of scope for follow-up research. Like all study groups and kindred organisations, we rely heavily on the willing co-operation and voluntary efforts of our members. ADSG is indeed fortunate in this regard. We are very grateful to Ruth Marriott for the design of our windcheater logo (see p.52 ). We were an extremely smart crew in white logo on navy at the recent Melbourne Wildflower Show. I The Daisy display made quite an impact and we have Maureen Schaumann to thank for the inspirational concept of "Uses of Daisies". One of the projects was undertaken in great secrecy. The only clue we had was that Maureen was working away with wood. At the unveiling we were stunned to see beautifully created necklaces designed from Cassinia aculeafa and C.laevis. What patience and creativity! Judy and Maureen supplied the range of dried flowers and Peggy MacAllister, Melbourne's Master Gardener, brought along a number of floral arrangements, a few of her own and some of her daughter's. -

Alpine Sphagnum Bogs and Associated Fens
Alpine Sphagnum Bogs and Associated Fens A nationally threatened ecological community Environment Protection and Biodiversity Conservation Act 1999 Policy Statement 3.16 This brochure is designed to assist land managers, owners and occupiers to identify, assess and manage the Alpine Sphagnum Bogs and Associated Fens, an ecological community listed under Australia’s national environment law, the Environment Protection and Biodiversity Conservation Act 1999 (EPBC Act). The brochure is a companion document to the listing advice which can be found at the Australian Government’s Species Profile and Threats Database (SPRAT). Please go to the Alpine Sphagnum Bogs and Associated Fens ecological community profile in SPRAT, then click on the ‘Details’ link: www.environment.gov.au/cgi-bin/sprat/public/publiclookupcommunities.pl • The Alpine Sphagnum Bogs and Associated Fens ecological community is found in small pockets in the high country of Tasmania, Victoria, New South Wales and the Australian Capital Territory. • The Alpine Sphagnum Bogs and Associated Fens ecological community can usually be defined by the presence or absence of sphagnum moss. • Long term conservation and restoration of this ecological community is essential in order to protect vital inland water resources. • Implementing favourable land use and management practices is encouraged at sites containing this ecological community. Disclaimer The contents of this document have been compiled using a range of source materials. This document is valid as at August 2009. The Commonwealth Government is not liable for any loss or damage that may be occasioned directly or indirectly through the use of or reliance on the contents of the document. © Commonwealth of Australia 2009 This work is copyright. -

Australian Plants Society South East NSW Group
Australian Plants Society South East NSW Group Newsletter 115 February 2016 Corymbia maculata Spotted Gum and Macrozamia communis Burrawang Contacts: President, Margaret Lynch, [email protected] Secretary, Michele Pymble, [email protected] Newsletter editor, John Knight, [email protected] Next Meeting 10.00am SATURDAY 5th March 2016 Eurobodalla Regional Botanic Gardens Plant Adaptations a walk and talk with a difference After a morning cuppa at the Friends shelter in the picnic area Margaret Lynch will lead an easy walk along the limited mobility track taking in the variety of display gardens including the sensory, rainforest and sandstone gardens. This is an ideal area to look closely at the diversity of characteristics in our regional plants. Variations in things such as form, texture, colour and smell of leaves, flowers and fruits often give a clue as to how plants grow and survive in different and often challenging environments. Come and join the discussion of what grows where and why and maybe discover what may do well at home for you. Following the walk there will be an opportunity to visit the propagation and nursery area for a behind the scenes look. Gardens manager, Michael Anlezark will outline the current workings of the area and the exciting future directions proposed for the Gardens. Lunch can either be the usual BYO picnic style or purchased at the Gardens café. The afternoon will be free to either stroll to the arboretum or browse the range of plants available for purchase from the plant sales area. As usual sensible footwear, hat, sunscreen, insect repellent and water are advisable. -

These De Doctorat De L'universite Paris-Saclay
NNT : 2016SACLS250 THESE DE DOCTORAT DE L’UNIVERSITE PARIS-SACLAY, préparée à l’Université Paris-Sud ÉCOLE DOCTORALE N° 567 Sciences du Végétal : du Gène à l’Ecosystème Spécialité de doctorat (Biologie) Par Mlle Nour Abdel Samad Titre de la thèse (CARACTERISATION GENETIQUE DU GENRE IRIS EVOLUANT DANS LA MEDITERRANEE ORIENTALE) Thèse présentée et soutenue à « Beyrouth », le « 21/09/2016 » : Composition du Jury : M., Tohmé, Georges CNRS (Liban) Président Mme, Garnatje, Teresa Institut Botànic de Barcelona (Espagne) Rapporteur M., Bacchetta, Gianluigi Università degli Studi di Cagliari (Italie) Rapporteur Mme, Nadot, Sophie Université Paris-Sud (France) Examinateur Mlle, El Chamy, Laure Université Saint-Joseph (Liban) Examinateur Mme, Siljak-Yakovlev, Sonja Université Paris-Sud (France) Directeur de thèse Mme, Bou Dagher-Kharrat, Magda Université Saint-Joseph (Liban) Co-directeur de thèse UNIVERSITE SAINT-JOSEPH FACULTE DES SCIENCES THESE DE DOCTORAT DISCIPLINE : Sciences de la vie SPÉCIALITÉ : Biologie de la conservation Sujet de la thèse : Caractérisation génétique du genre Iris évoluant dans la Méditerranée Orientale. Présentée par : Nour ABDEL SAMAD Pour obtenir le grade de DOCTEUR ÈS SCIENCES Soutenue le 21/09/2016 Devant le jury composé de : Dr. Georges TOHME Président Dr. Teresa GARNATJE Rapporteur Dr. Gianluigi BACCHETTA Rapporteur Dr. Sophie NADOT Examinateur Dr. Laure EL CHAMY Examinateur Dr. Sonja SILJAK-YAKOVLEV Directeur de thèse Dr. Magda BOU DAGHER KHARRAT Directeur de thèse Titre : Caractérisation Génétique du Genre Iris évoluant dans la Méditerranée Orientale. Mots clés : Iris, Oncocyclus, région Est-Méditerranéenne, relations phylogénétiques, status taxonomique. Résumé : Le genre Iris appartient à la famille des L’approche scientifique est basée sur de nombreux Iridacées, il comprend plus de 280 espèces distribuées outils moléculaires et génétiques tels que : l’analyse de à travers l’hémisphère Nord. -

Chromosomal and Molecular Evolution in Brachyscome (Astereae)
Kobe University Repository : Kernel タイトル Chromosomal and molecular evolution in Brachyscome (Astereae) Title 著者 Watanabe, Kuniaki / Denda, Tetsuo / Suzuki, Yohei / Kosuge, Keiko / Author(s) Ito, Motomi / Philip S. Short / Yahara, Tahara 掲載誌・巻号・ページ In D. J. N. Hind and H. Beentje (eds.) Compositae: Systematics,1:705- Citation 722 刊行日 1996 Issue date 資源タイプ Journal Article / 学術雑誌論文 Resource Type 版区分 publisher Resource Version 権利 Rights DOI JaLCDOI URL http://www.lib.kobe-u.ac.jp/handle_kernel/90001483 PDF issue: 2021-10-05 Watanabe, K., Denda, T., Suzuki, Y., Kosuge, K., Ito, M., Short, P.S. and Yahara, T. (1996). Chromosom~l and molecular evolution in the genus Brachyscome (Astereae). In D.l.N. Hind & H.J. Beentje (eds). Compositae: Systematics. Proceedings of the International Compositae Conference, Kew, 1994. (DJ.N. Hind, Editor-in-Chief), vol. 1. pp. 705-722. Royal Botanic Gardens, Kew. 47. CHROMOSOMAL AND MOLECULAR EVOL-UTION IN THE GENUS BRACHYSCOME (ASTEREAE) i i 2 i KUNIAKI WATANABE , TETSUO DENDA , YOHEI SUZUKI , KEIKO KOSUGE ', MOTOMI 2 3 4 IT0 , PHILIP S. SHORT AND TETSUKAZU YAHARA I Department of Biology, Faculty of Science, Kobe University, Tsurukabuto, 1-2-1, Kobe, 657, JAPAN 2 Department of Biology, Faculty of Science, Chiba University, Chiba, 260, JAPAN 3 National Herbarium of Victoria, Birdwood Avenue, South Yarra, Victoria, 3141, AUSTRALIA 4 Department of Biology, Faculty of Science, Kyushu University, Fukuoka, 812, JAPAN Abstract Intrageneric circumscription, interspecific relationships and chromosomal and molecular evolution of the Australian Brachyscome were examined using data from restriction site analysis of chloroplast DNA, karyotype analysis and DNA sequence analysis of the alcohol dehydrogenase gene (adh). -

The Murray–Darling Basin Basin Animals and Habitat the Basin Supports a Diverse Range of Plants and the Murray–Darling Basin Is Australia’S Largest Animals
The Murray–Darling Basin Basin animals and habitat The Basin supports a diverse range of plants and The Murray–Darling Basin is Australia’s largest animals. Over 350 species of birds (35 endangered), and most diverse river system — a place of great 100 species of lizards, 53 frogs and 46 snakes national significance with many important social, have been recorded — many of them found only in economic and environmental values. Australia. The Basin dominates the landscape of eastern At least 34 bird species depend upon wetlands in 1. 2. 6. Australia, covering over one million square the Basin for breeding. The Macquarie Marshes and kilometres — about 14% of the country — Hume Dam at 7% capacity in 2007 (left) and 100% capactiy in 2011 (right) Narran Lakes are vital habitats for colonial nesting including parts of New South Wales, Victoria, waterbirds (including straw-necked ibis, herons, Queensland and South Australia, and all of the cormorants and spoonbills). Sites such as these Australian Capital Territory. Australia’s three A highly variable river system regularly support more than 20,000 waterbirds and, longest rivers — the Darling, the Murray and the when in flood, over 500,000 birds have been seen. Australia is the driest inhabited continent on earth, Murrumbidgee — run through the Basin. Fifteen species of frogs also occur in the Macquarie and despite having one of the world’s largest Marshes, including the striped and ornate burrowing The Basin is best known as ‘Australia’s food catchments, river flows in the Murray–Darling Basin frogs, the waterholding frog and crucifix toad. bowl’, producing around one-third of the are among the lowest in the world. -

Annual Operations Plan Barwon-Darling 2019-20 Acronym Definition
Annual Operations Plan Barwon-Darling 2019-20 Acronym Definition AWD Available Water Determination Contents BLR Basic Landholder Rights BoM Bureau of Meteorology CWAP Critical Water Advisory Introduction 2 Panel The Barwon-Darling river system 2 CWTAG Critical Water Technical Unregulated system flow 3 Advisory Group Rainfall trends 3 DPI CDI Department of Primary Water users in the valley 4 Industries - Combined Drought Indicator Water availability 5 DPIE EES Department of Planning, Current drought conditions 6 Industry and Environment Resource assessment in the Northern regulated valleys 7 - Environment, Energy & Science Water resource forecast 9 DPI Department of Primary Barwon-Darling - past 24 month rainfall 9 Fisheries Industries - Fisheries Northern NSW River Systems - past 24 month flows 10 DPIE Department of Planning, Water Industry and Environment Weather forecast - 3 month BoM forecast 12 - Water Barwon-Darling flow 12 FSL Full Supply Level Annual operations 13 HS High Security Barwon-Darling flow class map 13 IRG Incident Response Guide Scenarios 14 ISEPP Infrastructure State Environmental Planning Projects 14 Policy LGA Local Government Areas ROSCCo River Operations Stakeholder Consultation Committee D&S Domestic and Stock vTAG Valley Technical Advisory Group Introduction This plan considers the current volume of water in storages of the tributary catchments and weather forecasts. This plan may be updated as a result significant changes to weather patterns. This year’s plan outlines WaterNSW’s response to the drought in the Barwon-Darling Valley including: • Identification of critical dates. • Our operational response. The NSW Department of Planning, Industry and Environment’s Extreme Events Policy and Incident Response Guides outlines the 4 stages of drought.