Clinical Trials Watch
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Shexiang Baoxin Pill, a Traditional Chinese Herbal Formula, Rescues the Cognitive Impairments in APP/ PS1 Transgenic Mice
ORIGINAL RESEARCH published: 14 July 2020 doi: 10.3389/fphar.2020.01045 Shexiang Baoxin Pill, a Traditional Chinese Herbal Formula, Rescues the Cognitive Impairments in APP/ PS1 Transgenic Mice † † Wei-Hui Hu 1,2,3 , Shing-Hung Mak 1,2 , Zhong-Yu Zheng 1,2, Ying-Jie Xia 1,2, Miranda Li Xu 1,2, Ran Duan 1,2,3, Tina Ting-Xia Dong 1,2,3, Shao-Ping Li 4, Chang-Sen Zhan 5,6, Xiao-Hui Shang 5,6 and Karl Wah-Keung Tsim 1,2,3* 1 Shenzhen Key Laboratory of Edible and Medicinal Bioresources, HKUST Shenzhen Research Institute, Shenzhen, China, 2 Division of Life Science and Center for Chinese Medicine and State Key Laboratory of Molecular Neuroscience, The Hong Kong University of Science and Technology, Hong Kong, Hong Kong, 3 Joint Laboratory of Guangdong Province and Hong Kong Region on Marine Bioresource Conservation and Exploitation, College of Marine Sciences, South China Agricultural University, Guangzhou, China, 4 Institute of Chinese Medical Sciences, University of Macau, Macau, Macau, 5 Shanghai Edited by: Engineering Research Center for Innovation of Solid Preparation of TCM, Shanghai, China, 6 Shanghai Hutchison Qiaobing Huang, Pharmaceuticals Ltd., Shanghai, China Southern Medical University, China Reviewed by: Background: Shexiang Baoxin Pill (SBP), a formulated traditional Chinese medicine Bing-Xing Pan, Nanchang University, China (TCM), has been widely used to treat cardiovascular diseases for years. This herbal Wenda Xue, mixture has been shown to promote differentiation of cultured neuronal cells. Here, we Nanjing University of Chinese aimed to investigate the effects of SBP in attenuating cognitive impairment in APP/PS1 Medicine, China *Correspondence: transgenic mice. -

The “Rights” of Precision Drug Development for Alzheimer's Disease
Cummings et al. Alzheimer's Research & Therapy (2019) 11:76 https://doi.org/10.1186/s13195-019-0529-5 REVIEW Open Access The “rights” of precision drug development for Alzheimer’s disease Jeffrey Cummings1*, Howard H. Feldman2 and Philip Scheltens3 Abstract There is a high rate of failure in Alzheimer’s disease (AD) drug development with 99% of trials showing no drug- placebo difference. This low rate of success delays new treatments for patients and discourages investment in AD drug development. Studies across drug development programs in multiple disorders have identified important strategies for decreasing the risk and increasing the likelihood of success in drug development programs. These experiences provide guidance for the optimization of AD drug development. The “rights” of AD drug development include the right target, right drug, right biomarker, right participant, and right trial. The right target identifies the appropriate biologic process for an AD therapeutic intervention. The right drug must have well-understood pharmacokinetic and pharmacodynamic features, ability to penetrate the blood-brain barrier, efficacy demonstrated in animals, maximum tolerated dose established in phase I, and acceptable toxicity. The right biomarkers include participant selection biomarkers, target engagement biomarkers, biomarkers supportive of disease modification, and biomarkers for side effect monitoring. The right participant hinges on the identification of the phase of AD (preclinical, prodromal, dementia). Severity of disease and drug mechanism both have a role in defining the right participant. The right trial is a well-conducted trial with appropriate clinical and biomarker outcomes collected over an appropriate period of time, powered to detect a clinically meaningful drug-placebo difference, and anticipating variability introduced by globalization. -

Current Research Efforts in the Prevention and Treatment of Alzheimer’S Disease (AD) and Related Dementias
Current Research efforts in the prevention and treatment of Alzheimer’s Disease (AD) and Related Dementias What’s new? 1. New and More effective Biomarkers 2. New Diagnostic Framework : • New “A/T/N” Framework • New Framework conceptualizes AD as a continuum from pathophysiological, biomarker and clinical perspectives. 3. LATE (Limbic-predominant, Age-related, TDP-43 Encephalopathy) looks like Alzheimer’s disease 4. Alzheimer’s pathophysiology starts decades prior to clinical disease! 1. New and More Effective Biomarkers AD Pathology • Alzheimer’s Disease consists of two main features: • Senile plaques (β-amyloid)- earliest pathological event! • Neurofibrillary tangles (hyperphosphorylated tau)- primary culprit in cells death • Mechanism: • β-amyloid hyperphosphorylated tau tau spreads throughout the brain from neuron to neuron (Pooler et al, 2013). Cell Death Biomarkers Budson & Solomon, Practical Neurology 2012;12:88–96 PET Amyloid Imaging was approved since 2012 • Use when knowing that AD pathology is present in symptomatic patient would change management. • May detect amyloid plaques in asymptomatic patients who may not develop disease for 10-15+ years • Not paid for by Medicare or other insurance companies • Can obtain through Veterans Affairs hospitals, clinical trials/research studies, and self-pay. • Will have broader use when disease modifying therapies are available. Alzheimer’s Disease Non-AD dementia 65 year old MoCA 21 From Budson & Solomon, 2016 PET tau imaging : FDA approved May 2020 • Use when knowing that AD pathology is present in symptomatic patient would change management. • Will detect AD tau tangles in symptomatic patients and should correlate with symptoms • May detect other types of tau tangles in other dementias (not yet clear) • Not paid for by Medicare or other insurance companies • Can obtain through Veterans Affairs hospitals, clinical trials/research studies, and self-pay. -

Drug Candidates in Clinical Trials for Alzheimer's Disease
Hung and Fu Journal of Biomedical Science (2017) 24:47 DOI 10.1186/s12929-017-0355-7 REVIEW Open Access Drug candidates in clinical trials for Alzheimer’s disease Shih-Ya Hung1,2 and Wen-Mei Fu3* Abstract Alzheimer’s disease (AD) is a major form of senile dementia, characterized by progressive memory and neuronal loss combined with cognitive impairment. AD is the most common neurodegenerative disease worldwide, affecting one-fifth of those aged over 85 years. Recent therapeutic approaches have been strongly influenced by five neuropathological hallmarks of AD: acetylcholine deficiency, glutamate excitotoxicity, extracellular deposition of amyloid-β (Aβ plague), formation of intraneuronal neurofibrillary tangles (NTFs), and neuroinflammation. The lowered concentrations of acetylcholine (ACh) in AD result in a progressive and significant loss of cognitive and behavioral function. Current AD medications, memantine and acetylcholinesterase inhibitors (AChEIs) alleviate some of these symptoms by enhancing cholinergic signaling, but they are not curative. Since 2003, no new drugs have been approved for the treatment of AD. This article focuses on the current research in clinical trials targeting the neuropathological findings of AD including acetylcholine response, glutamate transmission, Aβ clearance, tau protein deposits, and neuroinflammation. These investigations include acetylcholinesterase inhibitors, agonists and antagonists of neurotransmitter receptors, β-secretase (BACE) or γ-secretase inhibitors, vaccines or antibodies targeting Aβ clearance or tau protein, as well as anti-inflammation compounds. Ongoing Phase III clinical trials via passive immunotherapy against Aβ peptides (crenezumab, gantenerumab, and aducanumab) seem to be promising. Using small molecules blocking 5-HT6 serotonin receptor (intepirdine), inhibiting BACE activity (E2609, AZD3293, and verubecestat), or reducing tau aggregation (TRx0237) are also currently in Phase III clinical trials. -
A New Analysis of the Phase II Alzheimer's
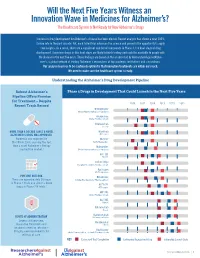
Will the Next Five Years Witness an Innovation Wave in Medicines for Alzheimer’s? The Healthcare System Is Not Ready for New Alzheimer’s Drugs Success in drug development for Alzheimer’s disease has been elusive. Recent analysis has shown a near 100% failure rate in the past decade. Yet, each failed trial advances the science and presents the opportunity to apply new insights. As a result, there are a significant number of compounds in Phase 3, the final stage of drug development. Seventeen drugs in this final stage are likely to finish testing and could be available to people with the disease in the next five years. These findings are based on the analysis led by ResearchersAgainstAlzhei- mer’s, a global network of leading Alzheimer’s researchers at top academic institutions and corporations. Our analysis leads us to be cautiously optimistic that innovative treatments are within our reach. We need to make sure the healthcare system is ready. Understanding the Alzheimer’s Drug Development Pipeline Robust Alzheimer’s Phase 3 Drugs in Development That Could Launch in the Next Five Years Pipeline Offers Promise for Treatment – Despite 2016 2017 2018 2019 2020 2021 Recent Track Record Brexpiprazole Otsuka Pharmaceuticals, H. Lundbeck Aripiprazole Otsuka Pharmaceuticals Solanezumab Eli Lilly MORE THAN A DECADE SINCE A NOVEL Masitinib ALZHEIMER’S DRUG WAS APPROVED AB Science Namenda was approved by TRx0237 the FDA in 2003, marking the last TauRx Therapeutics time a novel Alzheimer’s therapy Idalopirdine reached the market.1 Otsuka Pharmaceuticals, H. Lundbeck RVT-101 Axovant Sodium Oligo Shanghai Greenvalley Pharmaceuticals Azeliragon vTv Therapeutics PIPELINE OUTLOOK Nilvadipine There are approximately 50 drugs Astellas Pharma, Archer Pharmaceuticals in Phase 2 trials and about a dozen 2 ALZT-0P1 drugs in Phase 2/3 trials. -

Randomized Trial of Verubecestat for Mild-To-Moderate Alzheimer's Disease
The new england journal of medicine Original Article Randomized Trial of Verubecestat for Mild-to-Moderate Alzheimer’s Disease Michael F. Egan, M.D., James Kost, Ph.D., Pierre N. Tariot, M.D., Paul S. Aisen, M.D., Jeffrey L. Cummings, M.D., Sc.D., Bruno Vellas, M.D., Ph.D., Cyrille Sur, Ph.D., Yuki Mukai, M.D., Tiffini Voss, M.D., Christine Furtek, B.S., Erin Mahoney, B.A., Lyn Harper Mozley, Ph.D., Rik Vandenberghe, M.D., Ph.D., Yi Mo, Ph.D., and David Michelson, M.D. ABSTRACT BACKGROUND Alzheimer’s disease is characterized by the deposition of amyloid-beta (Aβ) plaques in From Merck Research Laboratories, Merck, the brain. Aβ is produced from the sequential cleavage of amyloid precursor protein Kenilworth, NJ (M.F.E., J.K., C.S., Y. Mu- kai, T.V., C.F., E.M., L.H.M., Y. Mo, D.M.); by β-site amyloid precursor protein–cleaving enzyme 1 (BACE-1) followed by Banner Alzheimer’s Institute, Phoenix, γ-secretase. Verubecestat is an oral BACE-1 inhibitor that reduces the Aβ level in the AZ (P.N.T.); University of Southern Cali- cerebrospinal fluid of patients with Alzheimer’s disease. fornia, San Diego (P.S.A.); Cleveland Clinic Lou Ruvo Center for Brain Health, METHODS Las Vegas (J.L.C.); Gerontopole, INSERM We conducted a randomized, double-blind, placebo-controlled, 78-week trial to evalu- Unité 1027, Alzheimer’s Disease Research and Clinical Center, Toulouse University ate verubecestat at doses of 12 mg and 40 mg per day, as compared with placebo, in Hospital, Toulouse, France (B.V.); and patients who had a clinical diagnosis of mild-to-moderate Alzheimer’s disease. -

Aducanumab for Alzheimer's Disease
Aducanumab for Alzheimer’s Disease: Effectiveness and Value Draft Evidence Report May 5, 2021 Prepared for ©Institute for Clinical and Economic Review, 2021 AUTHORS: Grace A. Lin, MD Associate Professor of Medicine and Health Policy University of California, San Francisco Melanie D. Whittington, PhD, MS Associate Director of Health Economics Institute for Clinical and Economic Review Patricia G. Synnott, MS, MALD Senior Manager, CEA Registry & Global Health Initiatives Center for the Evaluation of Value and Risk in Health Avery McKenna Research Assistant II, Evidence Synthesis Institute for Clinical and Economic Review Jon Campbell, PhD, MS Senior Vice President for Health Economics Institute for Clinical and Economic Review Steven D. Pearson, MD, MSc President Institute for Clinical and Economic Review David M. Rind, MD, MSc Chief Medical Officer Institute for Clinical and Economic Review DATE OF PUBLICATION: May 5, 2021 How to cite this document: Lin GA, Whittington MD, Synnott PG, McKenna A, Campbell J, Pearson SD, Rind DM. Aducanumab for Alzheimer’s Disease: Effectiveness and Value; Draft Evidence Report. Institute for Clinical and Economic Review, May 5, 2021. https://icer.org/assessment/alzheimers- disease-2021/. Grace A. Lin served as the lead author for the report. Patricia G. Synnott led the systematic review and authorship of the comparative clinical effectiveness section in collaboration with Avery McKenna and Emily Nhan. Melanie D. Whittington was responsible for the development of the cost-effectiveness model. Jon Campbell provided oversight of the cost-effectiveness analyses and ©Institute for Clinical and Economic Review, 2021 Page i Draft Evidence Report – Aducanumab for Alzheimer’s Disease developed the budget impact model. -

Downloaded in PDB Format from Protein Conducted Setting the Maximum Heavy Atom RMSD Data Bank (
Sarkar et al. Egyptian Journal of Medical Human Genetics (2021) 22:10 Egyptian Journal of Medical https://doi.org/10.1186/s43042-020-00127-8 Human Genetics RESEARCH Open Access Identification of the most potent acetylcholinesterase inhibitors from plants for possible treatment of Alzheimer’s disease: a computational approach Bishajit Sarkar1* , Sayka Alam1†, Tiluttoma Khan Rajib1†, Syed Sajidul Islam1†, Yusha Araf2 and Md. Asad Ullah1 Abstract Background: Being one of the rapidly growing dementia type diseases in the world, Alzheimer’s disease (AD) has gained much attention from researchers in the recent decades. Many hypotheses have been developed that describe different reasons for the development of AD. Among them, the cholinergic hypothesis depicts that the degradation of an important neurotransmitter, acetylcholine by the enzyme acetylcholinesterase (AChE), is responsible for the development of AD. Although, many anti-AChE drugs are already available in the market, their performance sometimes yields unexpected results. For this reason, research works are going on to find out potential anti-AChE agents both from natural and synthetic sources. In this study, 50 potential anti-AChE phytochemicals were analyzed using numerous tools of bioinformatics and in silico biology to find out the best possible anti-AChE agents among the selected 50 ligands through molecular docking, determination of the druglikeness properties, conducting the ADMET test, PASS and P450 site of metabolism prediction, and DFT calculations. Result: The predictions of this study suggested that among the selected 50 ligands, bellidifolin, naringenin, apigenin, and coptisine were the 4 best compounds with quite similar and sound performance in most of the experiments. -

Impact of Amyloid-Beta Changes on Cognitive Outcomes in Alzheimer's
Geerts et al. Alzheimer's Research & Therapy (2018) 10:14 DOI 10.1186/s13195-018-0343-5 RESEARCH Open Access Impact of amyloid-beta changes on cognitive outcomes in Alzheimer’s disease: analysis of clinical trials using a quantitative systems pharmacology model Hugo Geerts1,2* , Athan Spiros1 and Patrick Roberts1,3 Abstract Background: Despite a tremendous amount of information on the role of amyloid in Alzheimer’s disease (AD), almost all clinical trials testing this hypothesis have failed to generate clinically relevant cognitive effects. Methods: We present an advanced mechanism-based and biophysically realistic quantitative systems pharmacology computer model of an Alzheimer-type neuronal cortical network that has been calibrated with Alzheimer Disease Assessment Scale, cognitive subscale (ADAS-Cog) readouts from historical clinical trials and simulated the differential impact of amyloid-beta (Aβ40 and Aβ42) oligomers on glutamate and nicotinic neurotransmission. Results: Preclinical data suggest a beneficial effect of shorter Aβ forms within a limited dose range. Such a beneficial effect of Aβ40 on glutamate neurotransmission in human patients is absolutely necessary to reproduce clinical data on the ADAS-Cog in minimal cognitive impairment (MCI) patients with and without amyloid load, the effect of APOE genotype effect on the slope of the cognitive trajectory over time in placebo AD patients and higher sensitivity to cholinergic manipulation with scopolamine associated with higher Aβ in MCI subjects. We further derive a relationship between units of Aβ load in our model and the standard uptake value ratio from amyloid imaging. When introducing the documented clinical pharmacodynamic effects on Aβ levels for various amyloid-related clinical interventions in patients with low Aβ baseline, the platform predicts an overall significant worsening for passive vaccination with solanezumab, beta-secretase inhibitor verubecestat and gamma-secretase inhibitor semagacestat. -

AVN-211, Novel and Highly Selective 5-HT6 Receptor Small Molecule Antagonist, for the Treatment of Alzheimer's Disease Alexandre V
Subscriber access provided by University of Pennsylvania Libraries Article AVN-211, Novel and Highly Selective 5-HT6 Receptor Small Molecule Antagonist, for the Treatment of Alzheimer's Disease Alexandre V. Ivachtchenko, Yan Lavrovsky, and Yan A. Ivanenkov Mol. Pharmaceutics, Just Accepted Manuscript • DOI: 10.1021/acs.molpharmaceut.5b00830 • Publication Date (Web): 17 Feb 2016 Downloaded from http://pubs.acs.org on February 22, 2016 Just Accepted “Just Accepted” manuscripts have been peer-reviewed and accepted for publication. They are posted online prior to technical editing, formatting for publication and author proofing. The American Chemical Society provides “Just Accepted” as a free service to the research community to expedite the dissemination of scientific material as soon as possible after acceptance. “Just Accepted” manuscripts appear in full in PDF format accompanied by an HTML abstract. “Just Accepted” manuscripts have been fully peer reviewed, but should not be considered the official version of record. They are accessible to all readers and citable by the Digital Object Identifier (DOI®). “Just Accepted” is an optional service offered to authors. Therefore, the “Just Accepted” Web site may not include all articles that will be published in the journal. After a manuscript is technically edited and formatted, it will be removed from the “Just Accepted” Web site and published as an ASAP article. Note that technical editing may introduce minor changes to the manuscript text and/or graphics which could affect content, and all legal disclaimers and ethical guidelines that apply to the journal pertain. ACS cannot be held responsible for errors or consequences arising from the use of information contained in these “Just Accepted” manuscripts. -

Clinical Trials of New Drugs for Alzheimer Disease Li-Kai Huang1,2†, Shu-Ping Chao1,3† and Chaur-Jong Hu1,2,4,5*
Huang et al. Journal of Biomedical Science (2020) 27:18 https://doi.org/10.1186/s12929-019-0609-7 REVIEW Open Access Clinical trials of new drugs for Alzheimer disease Li-Kai Huang1,2†, Shu-Ping Chao1,3† and Chaur-Jong Hu1,2,4,5* Abstract Alzheimer disease (AD) accounts for 60–70% of dementia cases. Given the seriousness of the disease and continual increase in patient numbers, developing effective therapies to treat AD has become urgent. Presently, the drugs available for AD treatment, including cholinesterase inhibitors and an antagonist of the N-methyl-D-aspartate receptor, can only inhibit dementia symptoms for a limited period of time but cannot stop or reverse disease progression. On the basis of the amyloid hypothesis, many global drug companies have conducted many clinical trials on amyloid clearing therapy but without success. Thus, the amyloid hypothesis may not be completely feasible. The number of anti-amyloid trials decreased in 2019, which might be a turning point. An in-depth and comprehensive understanding of the contribution of amyloid beta and other factors of AD is crucial for developing novel pharmacotherapies. In ongoing clinical trials, researchers have developed and are testing several possible interventions aimed at various targets, including anti-amyloid and anti-tau interventions, neurotransmitter modification, anti-neuroinflammation and neuroprotection interventions, and cognitive enhancement, and interventions to relieve behavioral psychological symptoms. In this article, we present the current state of clinical trials for AD at clinicaltrials.gov.We reviewed the underlying mechanisms of these trials, tried to understand the reason why prior clinical trials failed, and analyzed the future trend of AD clinical trials. -

Spotlight on Axovant After Lundbeck's Alzheimer's Failure
September 23, 2016 Spotlight on Axovant after Lundbeck’s Alzheimer’s failure Madeleine Armstrong The phase III failure of Lundbeck’s idalopirdine is yet another example of just how difficult Alzheimer’s drug development is, and another nail in the coffin for the 5-HT6 antagonist class. It is therefore likely to be bad news for Lundbeck's rival Axovant, which uses the same approach with its own phase III project intepirdine. While some analysts were adamant that idalopirdine’s stumble was not a bad omen, it is hard to remain optimistic after the second late-stage collapse of a 5-HT6 antagonist this year – the other involved Pfizer’s PF-05212377, which was discontinued in February for lack of efficacy in a phase II trial. Axovant investors seemed inclined to agree: the company’s stock fell 12% yesterday. Meanwhile, Lundbeck was down as much as 15% today after yesterday's post-market release of data, and its partner Otsuka declined 3%. The analysts were in accord on one thing: this is likely the end of the road for idalopirdine, which will nevertheless limp on in two more phase III trials. These studies seem unlikely to return positive results after the Starshine trial failed to show a benefit on the primary endpoint, the ADAS-cog score, at either dose of idalopirdine – 30mg or 60mg on top of 10mg of Aricept against Aricept plus placebo. The project also failed to demonstrate an improvement on secondary endpoints. ABG Sundal Collier analysts were particularly scathing, writing: “We find nothing [in] the announcement to support any sort of optimism around idalopirdine being a potentially approvable drug.” The ongoing Starbeam study is testing idalopirdine at 10mg or 30mg on top of 10mg of Aricept, while Starbright evaluates 30mg or 60mg in combination with an unnamed acetylcholinesterase inhibitor – the same drug class as Aricept.