Spotlight on Axovant After Lundbeck's Alzheimer's Failure
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Upcoming Market Catalysts in Q4 2017
NEWS & ANALYSIS BIOBUSINESS BRIEFS MARKET WATCH Upcoming market catalysts in Q4 2017 Market catalysts in the fourth quarter of disease, and further results from a pivotal trial 2017 include top-line clinical trial results in Alzheimer disease are anticipated in late for tenapanor (developed by Ardelyx) for September. In light of the failure of other constipation-predominant irritable bowel 5HT6 receptor antagonists — including the syndrome (IBS-C) and intepirdine (developed recent failure of Lundbeck’s idalopirdine in by Axovant Sciences) for the treatment of several phase III trials in Alzheimer disease dementia with Lewy bodies, as well as the — the upcoming phase III data in Alzheimer expected approval of axicabtagene disease, along with the phase II data in ciloleucel (developed by Kite Pharma) in the dementia, will give important guidance United States for the treatment of relapsed/ to the future market opportunities for refractory aggressive B cell non-Hodgkin intepirdine. lymphoma (NHL). The Biologics License Application for Early in the quarter, Ardelyx expects Kite Pharma’s axicabtagene ciloleucel for the top-line results from T3MPO-2, a 6-month treatment of relapsed/refractory aggressive phase III study of tenapanor. This orally B cell NHL is under priority review, with a administered small molecule inhibits the Prescription Drug User Fee Act target action sodium–proton exchanger NHE3 in the date of 29 November 2017. This gastrointestinal tract, which increases the FDA-designated breakthrough therapy amount of sodium and fluid in the gut, consists of a patient’s peripheral blood thereby loosening stools. Results from a T lymphocytes that have been genetically previous 12-week phase III study known as engineered in vitro with chimeric antigen T3MPO-1 were statistically significant for receptors (CAR), enabling them to recognize the primary composite end point of response the tumour-expressed molecule CD19 rate, and seven out of eight secondary end after infusion back into the patient. -

Shexiang Baoxin Pill, a Traditional Chinese Herbal Formula, Rescues the Cognitive Impairments in APP/ PS1 Transgenic Mice
ORIGINAL RESEARCH published: 14 July 2020 doi: 10.3389/fphar.2020.01045 Shexiang Baoxin Pill, a Traditional Chinese Herbal Formula, Rescues the Cognitive Impairments in APP/ PS1 Transgenic Mice † † Wei-Hui Hu 1,2,3 , Shing-Hung Mak 1,2 , Zhong-Yu Zheng 1,2, Ying-Jie Xia 1,2, Miranda Li Xu 1,2, Ran Duan 1,2,3, Tina Ting-Xia Dong 1,2,3, Shao-Ping Li 4, Chang-Sen Zhan 5,6, Xiao-Hui Shang 5,6 and Karl Wah-Keung Tsim 1,2,3* 1 Shenzhen Key Laboratory of Edible and Medicinal Bioresources, HKUST Shenzhen Research Institute, Shenzhen, China, 2 Division of Life Science and Center for Chinese Medicine and State Key Laboratory of Molecular Neuroscience, The Hong Kong University of Science and Technology, Hong Kong, Hong Kong, 3 Joint Laboratory of Guangdong Province and Hong Kong Region on Marine Bioresource Conservation and Exploitation, College of Marine Sciences, South China Agricultural University, Guangzhou, China, 4 Institute of Chinese Medical Sciences, University of Macau, Macau, Macau, 5 Shanghai Edited by: Engineering Research Center for Innovation of Solid Preparation of TCM, Shanghai, China, 6 Shanghai Hutchison Qiaobing Huang, Pharmaceuticals Ltd., Shanghai, China Southern Medical University, China Reviewed by: Background: Shexiang Baoxin Pill (SBP), a formulated traditional Chinese medicine Bing-Xing Pan, Nanchang University, China (TCM), has been widely used to treat cardiovascular diseases for years. This herbal Wenda Xue, mixture has been shown to promote differentiation of cultured neuronal cells. Here, we Nanjing University of Chinese aimed to investigate the effects of SBP in attenuating cognitive impairment in APP/PS1 Medicine, China *Correspondence: transgenic mice. -

The 1,3,5-Triazine Derivatives As Innovative Chemical Family of 5-HT6 Serotonin Receptor Agents with Therapeutic Perspectives for Cognitive Impairment
International Journal of Molecular Sciences Article The 1,3,5-Triazine Derivatives as Innovative Chemical Family of 5-HT6 Serotonin Receptor Agents with Therapeutic Perspectives for Cognitive Impairment Gniewomir Latacz 1 , Annamaria Lubelska 1, Magdalena Jastrz˛ebska-Wi˛esek 2, Anna Partyka 2, Małgorzata Anna Mar´c 1, Grzegorz Satała 3, Daria Wilczy ´nska 2, Magdalena Kota ´nska 4, Małgorzata Wi˛ecek 1, Katarzyna Kami ´nska 1, Anna Wesołowska 2, Katarzyna Kie´c-Kononowicz 1 and Jadwiga Handzlik 1,* 1 Department of Technology and Biotechnology of Drugs, Medical College, Jagiellonian University, Medyczna 9, PL 30-688 Cracow, Poland 2 Department of Clinical Pharmacy, Medical College, Jagiellonian University, Medyczna 9, PL 30-688 Cracow, Poland 3 Department of Medicinal Chemistry Institute of Pharmacology, Polish Academy of Science, Sm˛etna12, PL 31-343 Cracow, Poland 4 Department of Pharmacodynamics, Faculty of Pharmacy, Medical College, Jagiellonian University, Medyczna 9, PL 30-688 Cracow, Poland * Correspondence: [email protected]; Tel.: +48-12-620-5580 Received: 27 May 2019; Accepted: 7 July 2019; Published: 12 July 2019 Abstract: Among serotonin receptors, the 5-HT6 subtype is the most controversial and the least known in the field of molecular mechanisms. The 5-HT6R ligands can be pivotal for innovative treatment of cognitive impairment, but none has reached pharmacological market, predominantly, due to insufficient “druglikeness” properties. Recently, 1,3,5-triazine-piperazine derivatives were identified as a new chemical family of potent 5-HT6R ligands. For the most active triazine 5-HT6R agents found (1–4), a wider binding profile and comprehensive in vitro evaluation of their drug-like parameters as well as behavioral studies and an influence on body mass in vivo were investigated within this work. -

The “Rights” of Precision Drug Development for Alzheimer's Disease
Cummings et al. Alzheimer's Research & Therapy (2019) 11:76 https://doi.org/10.1186/s13195-019-0529-5 REVIEW Open Access The “rights” of precision drug development for Alzheimer’s disease Jeffrey Cummings1*, Howard H. Feldman2 and Philip Scheltens3 Abstract There is a high rate of failure in Alzheimer’s disease (AD) drug development with 99% of trials showing no drug- placebo difference. This low rate of success delays new treatments for patients and discourages investment in AD drug development. Studies across drug development programs in multiple disorders have identified important strategies for decreasing the risk and increasing the likelihood of success in drug development programs. These experiences provide guidance for the optimization of AD drug development. The “rights” of AD drug development include the right target, right drug, right biomarker, right participant, and right trial. The right target identifies the appropriate biologic process for an AD therapeutic intervention. The right drug must have well-understood pharmacokinetic and pharmacodynamic features, ability to penetrate the blood-brain barrier, efficacy demonstrated in animals, maximum tolerated dose established in phase I, and acceptable toxicity. The right biomarkers include participant selection biomarkers, target engagement biomarkers, biomarkers supportive of disease modification, and biomarkers for side effect monitoring. The right participant hinges on the identification of the phase of AD (preclinical, prodromal, dementia). Severity of disease and drug mechanism both have a role in defining the right participant. The right trial is a well-conducted trial with appropriate clinical and biomarker outcomes collected over an appropriate period of time, powered to detect a clinically meaningful drug-placebo difference, and anticipating variability introduced by globalization. -

Current Research Efforts in the Prevention and Treatment of Alzheimer’S Disease (AD) and Related Dementias
Current Research efforts in the prevention and treatment of Alzheimer’s Disease (AD) and Related Dementias What’s new? 1. New and More effective Biomarkers 2. New Diagnostic Framework : • New “A/T/N” Framework • New Framework conceptualizes AD as a continuum from pathophysiological, biomarker and clinical perspectives. 3. LATE (Limbic-predominant, Age-related, TDP-43 Encephalopathy) looks like Alzheimer’s disease 4. Alzheimer’s pathophysiology starts decades prior to clinical disease! 1. New and More Effective Biomarkers AD Pathology • Alzheimer’s Disease consists of two main features: • Senile plaques (β-amyloid)- earliest pathological event! • Neurofibrillary tangles (hyperphosphorylated tau)- primary culprit in cells death • Mechanism: • β-amyloid hyperphosphorylated tau tau spreads throughout the brain from neuron to neuron (Pooler et al, 2013). Cell Death Biomarkers Budson & Solomon, Practical Neurology 2012;12:88–96 PET Amyloid Imaging was approved since 2012 • Use when knowing that AD pathology is present in symptomatic patient would change management. • May detect amyloid plaques in asymptomatic patients who may not develop disease for 10-15+ years • Not paid for by Medicare or other insurance companies • Can obtain through Veterans Affairs hospitals, clinical trials/research studies, and self-pay. • Will have broader use when disease modifying therapies are available. Alzheimer’s Disease Non-AD dementia 65 year old MoCA 21 From Budson & Solomon, 2016 PET tau imaging : FDA approved May 2020 • Use when knowing that AD pathology is present in symptomatic patient would change management. • Will detect AD tau tangles in symptomatic patients and should correlate with symptoms • May detect other types of tau tangles in other dementias (not yet clear) • Not paid for by Medicare or other insurance companies • Can obtain through Veterans Affairs hospitals, clinical trials/research studies, and self-pay. -

Drug Candidates in Clinical Trials for Alzheimer's Disease
Hung and Fu Journal of Biomedical Science (2017) 24:47 DOI 10.1186/s12929-017-0355-7 REVIEW Open Access Drug candidates in clinical trials for Alzheimer’s disease Shih-Ya Hung1,2 and Wen-Mei Fu3* Abstract Alzheimer’s disease (AD) is a major form of senile dementia, characterized by progressive memory and neuronal loss combined with cognitive impairment. AD is the most common neurodegenerative disease worldwide, affecting one-fifth of those aged over 85 years. Recent therapeutic approaches have been strongly influenced by five neuropathological hallmarks of AD: acetylcholine deficiency, glutamate excitotoxicity, extracellular deposition of amyloid-β (Aβ plague), formation of intraneuronal neurofibrillary tangles (NTFs), and neuroinflammation. The lowered concentrations of acetylcholine (ACh) in AD result in a progressive and significant loss of cognitive and behavioral function. Current AD medications, memantine and acetylcholinesterase inhibitors (AChEIs) alleviate some of these symptoms by enhancing cholinergic signaling, but they are not curative. Since 2003, no new drugs have been approved for the treatment of AD. This article focuses on the current research in clinical trials targeting the neuropathological findings of AD including acetylcholine response, glutamate transmission, Aβ clearance, tau protein deposits, and neuroinflammation. These investigations include acetylcholinesterase inhibitors, agonists and antagonists of neurotransmitter receptors, β-secretase (BACE) or γ-secretase inhibitors, vaccines or antibodies targeting Aβ clearance or tau protein, as well as anti-inflammation compounds. Ongoing Phase III clinical trials via passive immunotherapy against Aβ peptides (crenezumab, gantenerumab, and aducanumab) seem to be promising. Using small molecules blocking 5-HT6 serotonin receptor (intepirdine), inhibiting BACE activity (E2609, AZD3293, and verubecestat), or reducing tau aggregation (TRx0237) are also currently in Phase III clinical trials. -

2017 Medicines in Development for Alzheimer's Disease
2017 Medicines in Development for Alzheimer's Disease Alzheimer's Disease Product Name Sponsor Indication Development Phase ABBV-8E12 AbbVie Alzheimer's disease Phase II (anti-tau antibody) North Chicago, IL www.abbvie.com AC-1204 Accera mild to moderate Alzheimer's disease Phase III (glucose stimulant) Broomfield, CO www.accerapharma.com ACI-24 AC Immune Alzheimer's disease in Down Phase I (anti-Abeta vaccine) Lausanne, Switzerland syndrome patients www.acimmune.com ACI-35 AC Immune mild to moderate Alzheimer's disease Phase I (anti-pTau vaccine) Lausanne, Switzerland www.acimmune.com Janssen Research & Development www.janssen.com Raritan, NJ aducanumab (BIIB037) Biogen Alzheimer's disease (Fast Track) Phase III (amyloid beta mAb) Cambridge, MA www.biogen.com Neurimmune Zurich, Switzerland AGB101 AgenBio amnestic mild cognitive impairment Phase II completed (levetiracetam low-dose) Baltimore, MD in Alzheimer's disease www.agenebio.com Medicines in Development: Alzheimer's Disease | 2017 Update 1 Alzheimer's Disease Product Name Sponsor Indication Development Phase ALZ-801 Alzheon mild Alzheimer's disease Phase II (amyloid beta-protein inhibitor) Framingham, MA (homozygous APOE4/4 genotype) www.alzheon.com mild Alzheimer's disease Phase II (heterozygous APOE4 genotype) www.alzheon.com ALZT-OP1 AZTherapies Alzheimer's disease Phase III (amyloid beta-protein inhibitor/ Boston, MA www.aztherapies.com inflammation mediator inhibitor) AMG520/CNP520 Amgen Alzheimer's disease (Fast Track) Phase II/III (BACE1 protein inhibitor) Thousand Oaks, -

UNITED STATES SECURITIES and EXCHANGE COMMISSION Washington, D.C
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 8-K CURRENT REPORT Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 Date of Report (Date of Earliest Event Reported): January 8, 2018 Axovant Sciences Ltd. (Exact Name of Registrant as specified in its charter) Bermuda 001-37418 98-1333697 (State or Other Jurisdiction (Commission (IRS Employer of Incorporation) File Number) Identification No.) Suite 1, 3rd Floor 11-12 St. James’s Square London SW1Y 4LB, United Kingdom (Address of principal executive offices) Registrant’s telephone number, including area code: +44 203 318 9708 Not Applicable (Registrant’s name or former address, if change since last report) Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions: o Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) o Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) o Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) o Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter). Emerging growth company x If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. -
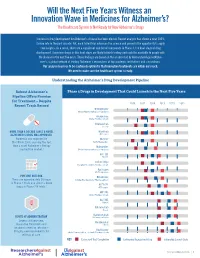
A New Analysis of the Phase II Alzheimer's
Will the Next Five Years Witness an Innovation Wave in Medicines for Alzheimer’s? The Healthcare System Is Not Ready for New Alzheimer’s Drugs Success in drug development for Alzheimer’s disease has been elusive. Recent analysis has shown a near 100% failure rate in the past decade. Yet, each failed trial advances the science and presents the opportunity to apply new insights. As a result, there are a significant number of compounds in Phase 3, the final stage of drug development. Seventeen drugs in this final stage are likely to finish testing and could be available to people with the disease in the next five years. These findings are based on the analysis led by ResearchersAgainstAlzhei- mer’s, a global network of leading Alzheimer’s researchers at top academic institutions and corporations. Our analysis leads us to be cautiously optimistic that innovative treatments are within our reach. We need to make sure the healthcare system is ready. Understanding the Alzheimer’s Drug Development Pipeline Robust Alzheimer’s Phase 3 Drugs in Development That Could Launch in the Next Five Years Pipeline Offers Promise for Treatment – Despite 2016 2017 2018 2019 2020 2021 Recent Track Record Brexpiprazole Otsuka Pharmaceuticals, H. Lundbeck Aripiprazole Otsuka Pharmaceuticals Solanezumab Eli Lilly MORE THAN A DECADE SINCE A NOVEL Masitinib ALZHEIMER’S DRUG WAS APPROVED AB Science Namenda was approved by TRx0237 the FDA in 2003, marking the last TauRx Therapeutics time a novel Alzheimer’s therapy Idalopirdine reached the market.1 Otsuka Pharmaceuticals, H. Lundbeck RVT-101 Axovant Sodium Oligo Shanghai Greenvalley Pharmaceuticals Azeliragon vTv Therapeutics PIPELINE OUTLOOK Nilvadipine There are approximately 50 drugs Astellas Pharma, Archer Pharmaceuticals in Phase 2 trials and about a dozen 2 ALZT-0P1 drugs in Phase 2/3 trials. -

Patent Application Publication ( 10 ) Pub . No . : US 2019 / 0192440 A1
US 20190192440A1 (19 ) United States (12 ) Patent Application Publication ( 10) Pub . No. : US 2019 /0192440 A1 LI (43 ) Pub . Date : Jun . 27 , 2019 ( 54 ) ORAL DRUG DOSAGE FORM COMPRISING Publication Classification DRUG IN THE FORM OF NANOPARTICLES (51 ) Int . CI. A61K 9 / 20 (2006 .01 ) ( 71 ) Applicant: Triastek , Inc. , Nanjing ( CN ) A61K 9 /00 ( 2006 . 01) A61K 31/ 192 ( 2006 .01 ) (72 ) Inventor : Xiaoling LI , Dublin , CA (US ) A61K 9 / 24 ( 2006 .01 ) ( 52 ) U . S . CI. ( 21 ) Appl. No. : 16 /289 ,499 CPC . .. .. A61K 9 /2031 (2013 . 01 ) ; A61K 9 /0065 ( 22 ) Filed : Feb . 28 , 2019 (2013 .01 ) ; A61K 9 / 209 ( 2013 .01 ) ; A61K 9 /2027 ( 2013 .01 ) ; A61K 31/ 192 ( 2013. 01 ) ; Related U . S . Application Data A61K 9 /2072 ( 2013 .01 ) (63 ) Continuation of application No. 16 /028 ,305 , filed on Jul. 5 , 2018 , now Pat . No . 10 , 258 ,575 , which is a (57 ) ABSTRACT continuation of application No . 15 / 173 ,596 , filed on The present disclosure provides a stable solid pharmaceuti Jun . 3 , 2016 . cal dosage form for oral administration . The dosage form (60 ) Provisional application No . 62 /313 ,092 , filed on Mar. includes a substrate that forms at least one compartment and 24 , 2016 , provisional application No . 62 / 296 , 087 , a drug content loaded into the compartment. The dosage filed on Feb . 17 , 2016 , provisional application No . form is so designed that the active pharmaceutical ingredient 62 / 170, 645 , filed on Jun . 3 , 2015 . of the drug content is released in a controlled manner. Patent Application Publication Jun . 27 , 2019 Sheet 1 of 20 US 2019 /0192440 A1 FIG . -

Randomized Trial of Verubecestat for Mild-To-Moderate Alzheimer's Disease
The new england journal of medicine Original Article Randomized Trial of Verubecestat for Mild-to-Moderate Alzheimer’s Disease Michael F. Egan, M.D., James Kost, Ph.D., Pierre N. Tariot, M.D., Paul S. Aisen, M.D., Jeffrey L. Cummings, M.D., Sc.D., Bruno Vellas, M.D., Ph.D., Cyrille Sur, Ph.D., Yuki Mukai, M.D., Tiffini Voss, M.D., Christine Furtek, B.S., Erin Mahoney, B.A., Lyn Harper Mozley, Ph.D., Rik Vandenberghe, M.D., Ph.D., Yi Mo, Ph.D., and David Michelson, M.D. ABSTRACT BACKGROUND Alzheimer’s disease is characterized by the deposition of amyloid-beta (Aβ) plaques in From Merck Research Laboratories, Merck, the brain. Aβ is produced from the sequential cleavage of amyloid precursor protein Kenilworth, NJ (M.F.E., J.K., C.S., Y. Mu- kai, T.V., C.F., E.M., L.H.M., Y. Mo, D.M.); by β-site amyloid precursor protein–cleaving enzyme 1 (BACE-1) followed by Banner Alzheimer’s Institute, Phoenix, γ-secretase. Verubecestat is an oral BACE-1 inhibitor that reduces the Aβ level in the AZ (P.N.T.); University of Southern Cali- cerebrospinal fluid of patients with Alzheimer’s disease. fornia, San Diego (P.S.A.); Cleveland Clinic Lou Ruvo Center for Brain Health, METHODS Las Vegas (J.L.C.); Gerontopole, INSERM We conducted a randomized, double-blind, placebo-controlled, 78-week trial to evalu- Unité 1027, Alzheimer’s Disease Research and Clinical Center, Toulouse University ate verubecestat at doses of 12 mg and 40 mg per day, as compared with placebo, in Hospital, Toulouse, France (B.V.); and patients who had a clinical diagnosis of mild-to-moderate Alzheimer’s disease. -

G-Protein-Coupled Receptors in CNS: a Potential Therapeutic Target for Intervention in Neurodegenerative Disorders and Associated Cognitive Deficits
cells Review G-Protein-Coupled Receptors in CNS: A Potential Therapeutic Target for Intervention in Neurodegenerative Disorders and Associated Cognitive Deficits Shofiul Azam 1 , Md. Ezazul Haque 1, Md. Jakaria 1,2 , Song-Hee Jo 1, In-Su Kim 3,* and Dong-Kug Choi 1,3,* 1 Department of Applied Life Science & Integrated Bioscience, Graduate School, Konkuk University, Chungju 27478, Korea; shofi[email protected] (S.A.); [email protected] (M.E.H.); md.jakaria@florey.edu.au (M.J.); [email protected] (S.-H.J.) 2 The Florey Institute of Neuroscience and Mental Health, The University of Melbourne, Parkville, VIC 3010, Australia 3 Department of Integrated Bioscience & Biotechnology, College of Biomedical and Health Science, and Research Institute of Inflammatory Disease (RID), Konkuk University, Chungju 27478, Korea * Correspondence: [email protected] (I.-S.K.); [email protected] (D.-K.C.); Tel.: +82-010-3876-4773 (I.-S.K.); +82-43-840-3610 (D.-K.C.); Fax: +82-43-840-3872 (D.-K.C.) Received: 16 January 2020; Accepted: 18 February 2020; Published: 23 February 2020 Abstract: Neurodegenerative diseases are a large group of neurological disorders with diverse etiological and pathological phenomena. However, current therapeutics rely mostly on symptomatic relief while failing to target the underlying disease pathobiology. G-protein-coupled receptors (GPCRs) are one of the most frequently targeted receptors for developing novel therapeutics for central nervous system (CNS) disorders. Many currently available antipsychotic therapeutics also act as either antagonists or agonists of different GPCRs. Therefore, GPCR-based drug development is spreading widely to regulate neurodegeneration and associated cognitive deficits through the modulation of canonical and noncanonical signals.