Adams Metal Oxide Catalysts for Solar Driven Water Splitting
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

University at Buffalo, Chemical and Biological Engineering February 2, 2021
University at Buffalo, Chemical and Biological Engineering February 2, 2021 CURRICULUM VITAE Gang Wu, Ph. D., Professor Department of Chemical and Biological Engineering University at Buffalo (UB), The State University of New York (SUNY), Buffalo, New York, USA E-mail: [email protected]; Phone: 716-645-8618 (office) ; 803-338-4924 (cell) Web: www.cbe.buffalo.edu/wu Education • 2004. Ph.D.: Environmental Engineering, Harbin Institute of Technology, Harbin, China. • 1999. M.S.: Applied Chemistry, Harbin Institute of Technology, Harbin, China. • 1997. B.S: Electrochemical Engineering, Harbin Institute of Technology, Harbin, China. Employment History • Aug 2020- present, Professor, University at Buffalo, SUNY, USA • Aug 2018-Aug 2020, Associate Professor, University at Buffalo, SUNY, USA • Aug 2014-Aug 2018, Assistant Professor, University at Buffalo, SUNY, USA • May 2010-Aug 2014, Staff Scientist, Los Alamos National Laboratory (LANL), USA • Jan 2008-May 2010, Postdoc, Los Alamos National Laboratory, USA • Feb 2006-Jan 2008, Postdoc, University of South Carolina, USA • Jan 2004-Jan 2006, Postdoc, Tsinghua University, Beijing, China Major Research Interest • Electrochemical Science and Engineering for Energy Technologies; • Electrocatalysis and photocatalysis for clean energy conversion: fuel cells, electrolyzers, CO2 reduction; electrosynthesis; • Electrochemical energy power sources for energy storage: batteries and supercapacitors; • Renewable fuel: NH3 (electrosynthesis, oxidation, and carking for H2 generation). Key Achievements and Recognition • Dr. Wu is internationally recognized as the leading researcher in the field of fuel cells and other sustainable electrochemical energy technologies. • Awarded more than $5.0 M in grant funding from federal agencies (DOE and NSF) since joining UB in August 2014. Those 14 projects (3 NSF and 11 DOE) focus on the development of advanced materials for electrochemical energy conversion and storage technologies such as fuel cells, water splitting, batteries, and renewable fuel (e.g., NH3). -

Spotlights on Our Sister Journals: Chemistryopen 5
On these pages, we feature aselection computer,click on any of the items to of the excellent work that has recently read the full article. Otherwise please been published in our sister journals. see the DOIs for easy online access If you are reading these pages on a through Wiley Online Library. Iodine Adducts A. Reinholdt, T. Vosch, J. Bendix* Modification of s-Donor Properties of TerminalCarbideLigands Investigated Through Carbide–Iodine AdductFormation To give and take:terminal ruthenium carbidecomplexes [(Cy P) X Ru C] (1;X= halide or pseudohalide), form charge-transfer 3 2 2 adducts with I2 exhibitinglarge variation in bond lengths and stretching frequencies. This showsthat the auxiliary ligand sphere on rutheniumenables control over the s-donor properties of car- bide ligands, elucidating their isolobal relationship with carbon monoxide. Angew.Chem. Int. Ed. DOI: 10.1002/anie.201606551 Density Functional Theory J. I. Schweizer, L. Meyer,A.Nadj, M. Diefenbach, M. C. Holthausen* Unraveling the Amine-Induced Disproportionation Reaction of Perchlorinated Silanes—A DFT Study Aneo twist:ADFT study on the amine-induceddisproportionation reactionofSi2Cl6 to neo-Si5Cl12 discloses astepwise rather than a concerted silylene insertion mechanism, which was generally ac- cepted for over half acentury. The resulting pictureappears gener- alizabletothe relatedchloride-induced chemistry recently explored (see graphic). Chem. Eur.J. DOI: 10.1002/chem.201602724 Aza-Diels–Alder Reactions Y. Kumatabara, S. Kaneko, S. Nakata,S.Shirakawa,* K. Maruoka Hydrogen-BondingCatalysis of TetraalkylammoniumSalts in an Aza-Diels–Alder Reaction Under observation:Apiperidine-derived tetraalkylammoniumsalt with anon-coordinating counteranion worked as an effective hydro- gen-bonding catalyst in an aza-Diels–Alder reactionofimines anda Danishefskydiene. -

Amatore CV English Personnal Data
C. Amatore, HonFRSC, HonFCSC Scientific Curriculum Vitæ French, Born December 1951, married, two Children 1. Academic Training o Education: • Undergraduate studies (1971-1974): Chemistry, Physics, Physical-Chemistry ; Ecole Normale Supérieure and University Paris 6, Paris, France, • Agrégation of Chemistry (1974) (highest professional professorship degree in France), o Doctorat d’Etat (1975-1979; cumulative grade equivalent to Ph.D. plus Habilitation; defended in December 1979); University Paris 7, Paris; performed under the guidance of Prof. Jean-Michel Savéant. o Post-Doctorate (1982-1983): Professor Jay K. Kochi, Indiana University at Bloomington, USA. 2. French Professorships 1974-1975 Research assistant, Department of Chemistry, Ecole Normale Supérieure (ENS), 1975-1984 CNRS Assistant Professor (AR, then CR), CNRS and University Paris 7, 1984-1990 CNRS Full Professor, first rank (DR2), CNRS and Department of Chemistry, ENS, 1990-2000 CNRS Full Professor, second rank (DR1), CNRS and Department of Chemistry, ENS, 2000-present CNRS Full Professor, first exceptional rank (DRCE1), CNRS and ENS, 2001-2004 Full Professor (former Louis Pasteur’s position), exceptional rank (PRCE1), University Pierre et Marie Curie (UPMC) and ENS, 2004-2007 Full Professor (former Louis Pasteur’s position), highest exceptional rank (PRCE2), University Pierre and Marie Curie (UPMC) and ENS, 2007-2017 Full Professor of the highest exceptional rank (DRCE CNRS) at ENS and UPMC, 2017-present Emeritus Professor of the highest exceptional rank (DRCE CNRS) at ENS -

Annual Report: CY2018 Chempubsoc Europe
1 Annual Report: CY2018 ChemPubSoc Europe Growth, change, awards, and sponsorship More scientists, more publications, more competition. As in the past, growth continued unabated worldwide in 2018 and is also reflected in the performance metrics of the ChemPubSoc Europe / Wiley-VCH publishing program, which now consists of 16 titles. The most recent product, ChemSystemsChem, was announced in Fall 2018. In 2018, the journals that are co-owned by ChemPubSoc Europe societies published nearly 9,000 articles (excluding contributions to the online publication ChemViews Magazine). Compared to the previous year, manuscript submissions grew by 12%, and the number of published articles rose by approximately 5%. A record number of full-text downloads, >8.9 million, was witnessed for the ChemPubSoc Europe journals, with increases in usage compared to the same time last year; notable among them ca. +20–30% for ChemCatChem, ChemElectroChem, ChemMedChem, and ChemPlusChem; and ca. +70% for ChemistrySelect and ChemPhotoChem. Approximately 3% of articles published in ChemPubSoc Europe's hybrid journals in 2018 were open access: ca. 10% at ChemBioChem and ca. 5% at ChemCatChem, Chemistry—A European Journal, ChemMedChem, and ChemPhysChem, respectively. ChemPubSoc Europe's gold open access journal, ChemistryOpen, experienced a ca. 47% growth in submissions compared to 2017. Generational change After the retirement of Peter Gölitz in November 2017, additional responsibility was taken on by Guido Herrmann (VP, Co-Managing Director Wiley-VCH, since April 1, 2017), Eva Wille (VP, Executive Director Wiley-VCH), Neville Compton (Editor-in-Chief of Angewandte Chemie and publisher for the GDCh journals), Haymo Ross (Editor-in-Chief of Chemistry—A European Journal and publisher of the ChemPubSoc Europe journals), Theresa Kueckmann (Editor-in-Chief of Chemistry—An Asian Journal and publisher for the Asian Chemical Editorial Society (ACES) journals), and Greta Heydenrych (development of new journals). -

Chemcatchem 11/2016
On these pages, we feature aselection computer,click on any of the items to of the excellent work that has recently read the full article. Otherwise please been published in our sister journals. see the DOIs for easy online access If you are reading these pages on a through Wiley Online Library. Gold Nanorod Analysis B. J. Plowman, N. P. Young, C. Batchelor-McAuley,R.G.Compton* Nanorod Aspect Ratios Determined by the Nano-Impact Technique Shapingup:The electrochemical determination of the aspectratio of gold nanorods on an individual basis through nano-impact ex- periments is demonstrated. The measured dimensions are in excel- lent agreement with electron microscopy results, establishing the use of nanoparticleimpact electrochemistry for the characterization of anisotropic nanomaterials. Angew.Chem. Int. Ed. DOI: 10.1002/anie.201602867 Graphene F. Wan, Y.-H. Li, D.-H. Liu, J.-Z. Guo, H.-Z. Sun, J.-P.Zhang,*X.-L. Wu* Alkali-Metal-Ion-Functionalized Graphene Oxide as aSuperior AnodeMaterial for Sodium-Ion Batteries Superior Na-storage properties of unreduced graphene oxide (GO) by asimple andscalable alkali-metal-ion (Li+,Na+,K+)-functional- ized process has been achieved. Different alkali metal ions play dif- ferent roles on adjusting the structure and Na-storage properties, and the GNa electrode exhibits much improved electrochemical properties in terms of higherrate performance and longer cycle sta- Chem. Eur.J. bility compared to GO, GLi, andGK(see figure). DOI: 10.1002/chem.201600660 Ruthenium Complexes K. Isozaki,* T. Yokoi, R. Yoshida, K. Ogata, D. Hashizume, N. Yasuda, K. Sadakane, H. Takaya,* M. Nakamura* Synthesis and Applicationsof (ONO Pincer)Ruthenium-Complex-BoundNorvalines ONO?OYes! (ONO pincer)ruthenium-complex-bound norvalines were successfully synthesized as anew type of bioorganometallic material. -

Eurjoceuropean Journal of Organic Chemistry Inorganic Chemistry
A Journal of A Journal of EurJIC European Journal of EurJOCEuropean Journal of Organic Chemistry Inorganic Chemistry 42/2019 44/2019 www.eurjic.org www.eurjoc.org A Journal of Front Cover Front Cover Felicitas Lips et al. Chutima Kuhakarn et al. Amido Silicon Chalcogenide Compounds with Unprecedented Cluster Cores Azide-Triggered Bicyclization of o-Alkynylisocyanobenzenes: Synthesis of Supported by www.chemeurj.org and Low Oxidation State Silicon Atoms Tetrazolo[1,5-a]quinolines 22/2019 2019-25/66 21/2019 Front Cover: A Journal of Front Cover: A Journal of Y. Guo et al. S. M. Eyrilmez, C. Köprülüoğlu et al. Capsule-Structured Copper–Zinc Catalyst for Highly Efficient Impressive Enrichment of Semiempirical Quantum Mechanics-Based Hydrogenation of Carbon Dioxide to Methanol Scoring Function: HSP90 Protein with 4541 Inhibitors and Decoys www.chemsuschem.org www.chemphyschem.org 21/2019 A Journal of Special Issue: A Journal of 11/2019 Front Cover: New Concepts in F. H. Lutter et al. Front Cover: Homogeneous Cobalt-Catalyzed Cross-Couplings and Electrophilic Aminations M. Konya et al. using Organozinc Pivalates Cationic Polymerization via Activation Catalysis of Alkoxyamines Using Photoredox Catalysts www.chemcatchem.org www.chemphotochem.org SpecialPhotopoly- Issue: merisation Supported by Front Cover: F. D. Toste, E. Gross et al. Flexible NO2-Functionalized N-Heterocyclic Carbene Monolayers on Au(111) Surface 22/2019 Front Cover: Gurcan Gunaydin, Safacan Kolemen, Gorkem Gunbas et al. Mitochondria-Targeting Selenophene-Modified BODIPY-Based Photosensitizers for the Treatment of Hypoxic Cancer Cells www.chemmedchem.org Wiley VCH Dienstag, 29.10.2019 1911 / 150392 [S. 894/894] 1 21/2019 22/2019 Front Cover: Front Cover: A Journal of S. -
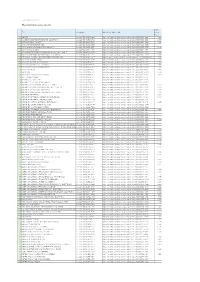
Last Updated 2017-10-3 Wiley Online Library Journals List 2018 Title
Last Updated 2017-10-3 Wiley Online Library Journals List 2018 2015 Title Journal DOI Wiley Online Library URL Impact Factor 1 ABACUS 10.1111/(ISSN)1467-6281 http://onlinelibrary.wiley.com/journal/10.1111/(ISSN)1467-6281 0.821 2 ACADEMIC EMERGENCY MEDICINE (ELECTRONIC) 10.1111/(ISSN)1553-2712 http://onlinelibrary.wiley.com/journal/10.1111/(ISSN)1553-2712 2.537 3 ACCOUNTING & FINANCE 10.1111/(ISSN)1467-629X http://onlinelibrary.wiley.com/journal/10.1111/(ISSN)1467-629X 0.927 4 ACCOUNTING PERSPECTIVES 10.1111/(ISSN)1911-3838 http://onlinelibrary.wiley.com/journal/10.1111/(ISSN)1911-3838 5 ACTA ANAESTHESIOLOGICA SCANDINAVICA 10.1111/(ISSN)1399-6576 http://onlinelibrary.wiley.com/journal/10.1111/(ISSN)1399-6576 2.049 6 ACTA ARCHAEOLOGICA 10.1111/(ISSN)1600-0390 http://onlinelibrary.wiley.com/journal/10.1111/(ISSN)1600-0390 7 ACTA GEOLOGICA SINICA (ENGLISH EDITION) (ELECTRONIC) 10.1111/(ISSN)1755-6724 http://onlinelibrary.wiley.com/journal/10.1111/(ISSN)1755-6724 1.996 8 ACTA NEUROLOGICA SCANDINAVICA (ELECTRONIC) 10.1111/(ISSN)1600-0404 http://onlinelibrary.wiley.com/journal/10.1111/(ISSN)1600-0404 2.559 9 ACTA OBSTETRICIA ET GYNECOLOGICA SCANDINAVICA 10.1111/(ISSN)1600-0412 http://obgyn.onlinelibrary.wiley.com/hub/journal/10.1111/(ISSN)1600-04 2.426 10 ACTA OPHTHALMOLOGICA 10.1111/(ISSN)1755-3768 http://onlinelibrary.wiley.com/journal/10.1111/(ISSN)1755-3768 3.032 11 ACTA PAEDIATRICA (ELECTRONIC) 10.1111/(ISSN)1651-2227 http://onlinelibrary.wiley.com/journal/10.1111/(ISSN)1651-2227 1.674 12 ACTA PHYSIOLOGICA (ELECTRONIC) 10.1111/(ISSN)1748-1716 -

2018 Journal Citation Reports Journals in the 2018 Release of JCR 2 Journals in the 2018 Release of JCR
2018 Journal Citation Reports Journals in the 2018 release of JCR 2 Journals in the 2018 release of JCR Abbreviated Title Full Title Country/Region SCIE SSCI 2D MATER 2D MATERIALS England ✓ 3 BIOTECH 3 BIOTECH Germany ✓ 3D PRINT ADDIT MANUF 3D PRINTING AND ADDITIVE MANUFACTURING United States ✓ 4OR-A QUARTERLY JOURNAL OF 4OR-Q J OPER RES OPERATIONS RESEARCH Germany ✓ AAPG BULL AAPG BULLETIN United States ✓ AAPS J AAPS JOURNAL United States ✓ AAPS PHARMSCITECH AAPS PHARMSCITECH United States ✓ AATCC J RES AATCC JOURNAL OF RESEARCH United States ✓ AATCC REV AATCC REVIEW United States ✓ ABACUS-A JOURNAL OF ACCOUNTING ABACUS FINANCE AND BUSINESS STUDIES Australia ✓ ABDOM IMAGING ABDOMINAL IMAGING United States ✓ ABDOM RADIOL ABDOMINAL RADIOLOGY United States ✓ ABHANDLUNGEN AUS DEM MATHEMATISCHEN ABH MATH SEM HAMBURG SEMINAR DER UNIVERSITAT HAMBURG Germany ✓ ACADEMIA-REVISTA LATINOAMERICANA ACAD-REV LATINOAM AD DE ADMINISTRACION Colombia ✓ ACAD EMERG MED ACADEMIC EMERGENCY MEDICINE United States ✓ ACAD MED ACADEMIC MEDICINE United States ✓ ACAD PEDIATR ACADEMIC PEDIATRICS United States ✓ ACAD PSYCHIATR ACADEMIC PSYCHIATRY United States ✓ ACAD RADIOL ACADEMIC RADIOLOGY United States ✓ ACAD MANAG ANN ACADEMY OF MANAGEMENT ANNALS United States ✓ ACAD MANAGE J ACADEMY OF MANAGEMENT JOURNAL United States ✓ ACAD MANAG LEARN EDU ACADEMY OF MANAGEMENT LEARNING & EDUCATION United States ✓ ACAD MANAGE PERSPECT ACADEMY OF MANAGEMENT PERSPECTIVES United States ✓ ACAD MANAGE REV ACADEMY OF MANAGEMENT REVIEW United States ✓ ACAROLOGIA ACAROLOGIA France ✓ -

2019 Selected Editions: SCIE,SSCI Selected Quartiles: 'Q1'
Journal Data Filtered By: Selected JCR Year: 2019 Selected Editions: SCIE,SSCI Selected Quartiles: 'Q1' Selected Category Scheme: WoS Rank Full Journal Title Journal Impact FactorJIF Quartile 1 CA-A CANCER JOURNAL FOR CLINICIANS 292.278 Q1 2 NEW ENGLAND JOURNAL OF MEDICINE 74.699 Q1 3 Nature Reviews Materials 71.189 Q1 4 NATURE REVIEWS DRUG DISCOVERY 64.797 Q1 5 LANCET 60.392 Q1 6 WHO Technical Report Series 59 Q1 7 NATURE REVIEWS MOLECULAR CELL BIOLOGY 55.47 Q1 8 Nature Reviews Clinical Oncology 53.276 Q1 9 NATURE REVIEWS CANCER 53.03 Q1 10 CHEMICAL REVIEWS 52.758 Q1 11 Nature Energy 46.495 Q1 12 JAMA-JOURNAL OF THE AMERICAN MEDICAL ASSOCIATION 45.54 Q1 13 REVIEWS OF MODERN PHYSICS 45.037 Q1 14 CHEMICAL SOCIETY REVIEWS 42.846 Q1 15 NATURE 42.778 Q1 16 SCIENCE 41.845 Q1 17 Nature Reviews Disease Primers 40.689 Q1 18 World Psychiatry 40.595 Q1 18 World Psychiatry 40.595 Q1 20 NATURE REVIEWS IMMUNOLOGY 40.358 Q1 21 NATURE MATERIALS 38.663 Q1 22 CELL 38.637 Q1 23 NATURE BIOTECHNOLOGY 36.558 Q1 24 NATURE MEDICINE 36.13 Q1 25 Living Reviews in Relativity 35.429 Q1 26 Nature Reviews Chemistry 34.953 Q1 27 NATURE REVIEWS MICROBIOLOGY 34.209 Q1 28 LANCET ONCOLOGY 33.752 Q1 29 NATURE REVIEWS NEUROSCIENCE 33.654 Q1 30 NATURE REVIEWS GENETICS 33.133 Q1 31 Annual Review of Astronomy and Astrophysics 32.963 Q1 32 JOURNAL OF CLINICAL ONCOLOGY 32.956 Q1 33 PROGRESS IN MATERIALS SCIENCE 31.56 Q1 34 Nature Nanotechnology 31.538 Q1 35 Nature Photonics 31.241 Q1 36 NATURE METHODS 30.822 Q1 37 Nature Catalysis 30.471 Q1 38 Energy & Environmental Science 30.289 -

Journal Impact Factor (JCR 2018)
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/323571463 2018 Journal Impact Factor (JCR 2018) Technical Report · March 2018 CITATIONS READS 0 36,968 1 author: Chunbiao Zhu Peking University 21 PUBLICATIONS 35 CITATIONS SEE PROFILE Some of the authors of this publication are also working on these related projects: Robust Saliency Detection via Fusing Foreground and Background Priors View project A Multilayer Backpropagation Saliency Detection Algorithm Based on Depth Mining View project All content following this page was uploaded by Chunbiao Zhu on 27 June 2018. The user has requested enhancement of the downloaded file. Journal Data Filtered By: Selected JCR Year: 2017 Selected Editions: SCIE,SSCI Selected Category Scheme: WoS Journal Eigenfactor Rank Full Journal Title Total Cites Impact Score 1 CA-A CANCER JOURNAL FOR CLINICIANS 28,839 244.585 0.066030 2 NEW ENGLAND JOURNAL OF MEDICINE 332,830 79.258 0.702000 3 LANCET 233,269 53.254 0.435740 4 CHEMICAL REVIEWS 174,920 52.613 0.265650 5 Nature Reviews Materials 3,218 51.941 0.015060 6 NATURE REVIEWS DRUG DISCOVERY 31,312 50.167 0.054410 7 JAMA-JOURNAL OF THE AMERICAN MEDICAL ASSOCIATION 148,774 47.661 0.299960 8 Nature Energy 5,072 46.859 0.020430 9 NATURE REVIEWS CANCER 50,407 42.784 0.079730 10 NATURE REVIEWS IMMUNOLOGY 39,215 41.982 0.085360 11 NATURE 710,766 41.577 1.355810 12 NATURE REVIEWS GENETICS 35,680 41.465 0.094300 13 SCIENCE 645,132 41.058 1.127160 14 CHEMICAL SOCIETY REVIEWS 125,900 40.182 0.275690 15 NATURE MATERIALS -

Liste Des Publications Équipe De Chimie Organique Pour Les Matériaux, La Biologie Et L’Optique Période 2013-2018
Liste des publications Équipe de Chimie Organique pour les Matériaux, la Biologie et l’Optique Période 2013-2018 -------------------------------------------------------------------------------------------------------------------------------------- Publications de 2018 Dyes and Pigments 2019, 163, 475–482. Jacquemin, Denis; Khelladi, Mustapha; De Nicola, Antoinette; Ulrich, Gilles Turning ESIPT-Based triazine fluorophores into dual emitters: From theory to experiment European Journal of Organic Chemistry (2018), Ahead of Print. , Ethynyl-Tolyl Extended 2- (2'-Hydroxyphenyl)benzoxazole Dyes: Solution and Solid-state Excited-State Intramolecular Proton Transfer (ESIPT) Emitters Munch, Maxime; Curtil, Mathieu; Verite, Pauline M.; Jacquemin, Denis; Massue, Julien; Ulrich, Gilles. Journal of Organic Chemistry 2018, 83, 14406-14418, Synthesis of Indolo[3,2-b]carbazole- Based Boron Complexes with Tunable Photophysical and Electrochemical Properties Mula, S.; Leclerc, N.; Leveque, P.; Retailleau, P.; Ulrich, G. Chemistry Letters 2018, 47, 1083-1089. Massue, J.; Jacquemin, D.; Ulrich, G. Molecular engineering of excited-state intramolecular proton transfer (ESIPT) dual and triple emitters. Dyes and Pigments 2019, 160, 915–922. Massue, J.; Felouat, A.; Curtil, M.; Vérité, P. M.; Jacquemin, D.; Ulrich, G. Solution and Solid-State Excited-State Intramolecular Proton Transfer (ESIPT) Emitters Incorporating Bis-Triethyl-or Triphenylsilylethynyl Units J. Mater. Chem. C 2018, 6 (37), 9925–9931. Huaulmé, Q.; Sutter, A.; Fall, S.; Jacquemin, D.; Lévêque, P.; Retailleau, P.; Ulrich, G.; Leclerc, N. Versatile Synthesis of α-Fused BODIPY Displaying Intense Absorption in the NIR Region and High Electron Affinity ChemistrySelect 2018, 3, 7674– 7679. Courrier, E.; Maret, C.; Charaoui-Boukerzaza, S.; Lambert, V.; De Nicola, A.; Muzuzu, W.; Ulrich, G. Raberin, H.; Flori, P.; Moine, B.; He, Z., Gain, P., Thuret G. -
General Information
General Information Dear Author, Thank you for choosing ChemPlusChem for your publication. Please follow this step-by-step guide to help us publish your manuscript as quickly as possible. This document contains the following information: 1 Revising Manuscripts ............................................................................................................................ 2 2 Accepted Article .................................................................................................................................... 3 3 Graphics ................................................................................................................................................ 4 4 Submitting Cover Artwork .................................................................................................................... 5 Visit our homepage or contact the Editorial Office if you have any additional questions: Phone (+49) 6201-606-305 E-mail: [email protected] Homepage: http://www.chempluschem.org We look forward to the publication of your article! With kind regards, ChemPlusChem Editorial Team ChemPlusChem is a ChemPubSoc Europe journal published by Wiley-VCH. You may wish to consider sister journals from the ChemPubSoc Europe program for future papers. Visit http://www.chempubsoc.eu for more information on these journals: Chemistry – A European Journal, European Journal of Organic Chemistry, European Journal of Inorganic Chemistry, Batteries & Supercaps, ChemBioChem, ChemCatChem, ChemElectroChem, ChemMedChem, ChemPhotoChem,