Insights on Alpha Lipoic and Dihydrolipoic Acids As Promising
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Review of Succimer for Treatment of Lead Poisoning
Review of Succimer for treatment of lead poisoning Glyn N Volans MD, BSc, FRCP. Department of Clinical Pharmacology, School of Medicine at Guy's, King's College & St Thomas' Hospitals, St Thomas' Hospital, London, UK Lakshman Karalliedde MB BS, DA, FRCA Consultant Medical Toxicologist, CHaPD (London), Health Protection Agency UK, Visiting Senior Lecturer, Division of Public Health Sciences, King's College Medical School, King's College , London Senior Research Collaborator, South Asian Clinical Toxicology Research Collaboration, Faculty of Medicine, Peradeniya, Sri Lanka. Heather M Wiseman BSc MSc Medical Toxicology Information Services, Guy’s and St Thomas’ NHS Foundation Trust, London SE1 9RT, UK. Contact details: Heather Wiseman Medical Toxicology Information Services Guy’s & St Thomas’ NHS Foundation Trust Mary Sheridan House Guy’s Hospital Great Maze Pond London SE1 9RT Tel 020 7188 7188 extn 51699 or 020 7188 0600 (admin office) Date 10th March 2010 succimer V 29 Nov 10.doc last saved: 29-Nov-10 11:30 Page 1 of 50 CONTENTS 1 Summary 2. Name of the focal point in WHO submitting or supporting the application 3. Name of the organization(s) consulted and/or supporting the application 4. International Nonproprietary Name (INN, generic name) of the medicine 5. Formulation proposed for inclusion 6. International availability 7. Whether listing is requested as an individual medicine or as an example of a therapeutic group 8. Public health relevance 8.1 Epidemiological information on burden of disease due to lead poisoning 8.2 Assessment of current use 8.2.1 Treatment of children with lead poisoning 8.2.2 Other indications 9. -

WO 2014/195872 Al 11 December 2014 (11.12.2014) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2014/195872 Al 11 December 2014 (11.12.2014) P O P C T (51) International Patent Classification: (74) Agents: CHOTIA, Meenakshi et al; K&S Partners | Intel A 25/12 (2006.01) A61K 8/11 (2006.01) lectual Property Attorneys, 4121/B, 6th Cross, 19A Main, A 25/34 (2006.01) A61K 8/49 (2006.01) HAL II Stage (Extension), Bangalore 560038 (IN). A01N 37/06 (2006.01) A61Q 5/00 (2006.01) (81) Designated States (unless otherwise indicated, for every A O 43/12 (2006.01) A61K 31/44 (2006.01) kind of national protection available): AE, AG, AL, AM, AO 43/40 (2006.01) A61Q 19/00 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, A01N 57/12 (2006.01) A61K 9/00 (2006.01) BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, AOm 59/16 (2006.01) A61K 31/496 (2006.01) DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (21) International Application Number: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, PCT/IB20 14/06 1925 KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, (22) International Filing Date: OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, 3 June 2014 (03.06.2014) SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, (25) Filing Language: English TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. -

The Toxicology of Mercury Current Research and Emerging Trends
Environmental Research 159 (2017) 545–554 Contents lists available at ScienceDirect Environmental Research journal homepage: www.elsevier.com/locate/envres The toxicology of mercury: Current research and emerging trends MARK ⁎ Geir Bjørklunda, , Maryam Dadarb, Joachim Mutterc, Jan Aasethd a Council for Nutritional and Environmental Medicine, Toften 24, 8610 Mo i Rana, Norway b Razi Vaccine and Serum Research Institute, Agricultural Research, Education and Extension Organization (AREEO), Karaj, Iran c Paracelsus Clinica al Ronc, Castaneda, Switzerland d Innlandet Hospital Trust and Inland Norway University of Applied Sciences, Elverum, Norway ARTICLE INFO ABSTRACT Keywords: Mercury (Hg) is a persistent bio-accumulative toxic metal with unique physicochemical properties of public Mercury health concern since their natural and anthropogenic diffusions still induce high risk to human and environ- Selenium mental health. The goal of this review was to analyze scientific literature evaluating the role of global concerns Thiols over Hg exposure due to human exposure to ingestion of contaminated seafood (methyl-Hg) as well as elemental Copper Hg levels of dental amalgam fillings (metallic Hg), vaccines (ethyl-Hg) and contaminated water and air (Hg Zinc chloride). Mercury has been recognized as a neurotoxicant as well as immunotoxic and designated by the World Toxicology Health Organization as one of the ten most dangerous chemicals to public health. It has been shown that the half- life of inorganic Hg in human brains is several years to several decades. Mercury occurs in the environment under different chemical forms as elemental Hg (metallic), inorganic and organic Hg. Despite the raising un- derstanding of the Hg toxicokinetics, there is still fully justified to further explore the emerging theories about its bioavailability and adverse effects in humans. -

Chelating Drug Therapy: an Update
Open Access Austin Journal of Genetics and Genomic Research Review Article Chelating Drug Therapy: An Update Vijay Kumar1, Ashok Kumar2*, Sandeep Kumar Singh1, Manoj Kumar3, Surendra Kumar2, Dinesh Abstract 4 5 Kumar and Ragni Singh Purpose: To study the clinical effects of metal toxicity and current 1Department of Neurology, SGPGIMS, India recommendations for management, including chelation therapy, are reviewed. 2Department of Medical Genetics, SGPGIMS, India 3Department of Microbiology, SGPGIMS, India Summary: Metals are essential to many biological processes, but excess 4Department of Chemistry, Dr. R.M.L. Avadh University, of it becomes hazardous to life. These are necessary for cell growth, electron India transport chain, several enzymatic activities and response of immune systems. 5Bheem Rao Ambedkar Bihar University, India They also serve as a cofactor for several enzymes. Chelation therapy is used for clinical management of the excess of metal. However, each metal requires *Corresponding author: Ashok Kumar, Department a specific chelation agent. A chelate is a compound form between metal and a of Medical Genetics, Sanjay Gandhi Post Graduate compound that contains two or more potential ligands. A promising Fe chelator Institute of Medical Sciences, Lucknow, India is Desferrioxamine (Desferal). Penicillamine and Trientine are uses for copper Received: March 12, 2015; Accepted: April 24, 2015; chelation. Meso-2,3-Dimercaptosuccinic Acid (DMSA) and 2,3-Dimercapto- Published: April 27, 2015 Propanesulphonate (DMPS) can be used as effective chelator of mercury. Dimercaprol, edetate calcium disodium, and succimer are the three agents primarily used for chelation of lead. Conclusion: Metal toxicity remains a significant public health concern. Elimination of elevated metal ions can be achieved by proper chelation agents. -

Changes in Tissue Gadolinium Biodistribution Measured in an Animal Model Exposed to Four Chelating Agents
Japanese Journal of Radiology (2019) 37:458–465 https://doi.org/10.1007/s11604-019-00835-1 ORIGINAL ARTICLE Changes in tissue gadolinium biodistribution measured in an animal model exposed to four chelating agents Türker Acar1,6 · Egemen Kaya2 · Mustafa Deniz Yoruk3 · Neslihan Duzenli4 · Recep Selim Senturk4 · Cenk Can4 · Lokman Ozturk3 · Canberk Tomruk5 · Yigit Uyanikgil5 · Frank J. Rybicki6 Received: 17 December 2018 / Accepted: 22 March 2019 / Published online: 30 March 2019 © Japan Radiological Society 2019 Abstract Purpose This study investigated the potential to reduce gadolinium levels in rodents after repetitive IV Gadodiamide admin- istration using several chelating agents. Materials and methods The following six groups of rats were studied. Group 1: Control; Group 2: Gadodiamide only; Group 3: Meso-2,3-Dimercaptosuccinic acid (DMSA) + Gadodiamide; Group 4: N-Acetyl-L-cysteine (NAC) + Gadodiamide; Group 5: Coriandrum sativum extract + Gadodiamide; and Group 6: Deferoxamine + Gadodiamide. Brain, kidney, and blood samples were evaluated via inductively coupled plasma mass spectrometry. The brain was also evaluated histologically. Results Kidney gadolinium levels in Groups 4 and 5 were approximately double that of Group 2 (p = 0.033 for each). There was almost no calcifcation in rat hippocampus for Group 4 rodents when compared with Groups 2, 3, 5 and 6. Conclusion Our preliminary study shows that excretion to the kidney has a higher propensity in NAC and Coriandrum sati- vum groups. It may be possible to change the distribution of gadolinium by administrating several agents. NAC may lower Gadodiamide-induced mineralization in rat hippocampus. Keywords Gadolinium deposition · Dimercaptosuccinic acid · N-Acetyl-L-cysteine · Coriandrum sativum · Deferoxamine Introduction However, the safety profle of gadolinium-based agents [1] was questioned after the discovery of nephrogenic sys- Gadolinium-based contrast agents (GBCA) have been safely temic fbrosis [2], a scleroderma-like disease characterized used in diagnostic radiology since the 1980s. -
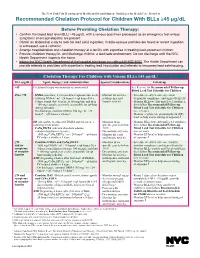
Recommended Chelation Protocol for Children with Blls ≥45 Μg/Dl
The New York City Department of Health and Mental Hygiene Guidelines for Health Care Providers Recommended Chelation Protocol for Children With BLLs ≥45 μg/dL Before Providing Chelation Therapy: • Confirm the blood lead level (BLL) ≥45 μg/dL with a venous specimen processed as an emergency test unless symptoms of encephalopathy are present. • Obtain an abdominal x-ray to look for lead solid ingestion; if radio-opaque particles are found or recent ingestion is witnessed, use a cathartic. • Arrange hospitalization and chelation therapy at a facility with expertise in treating lead-poisoned children. • Provide chelation therapy in, and discharge child to, a lead-safe environment. Do not discharge until the NYC Health Department inspects the home. • Inform the NYC Health Department of the hospital admission by calling 646-632-6002. The Health Department can provide referrals to providers with expertise in treating lead intoxication and referrals to temporary lead-safe housing. Chelation Therapy For Children with Venous BLLs ≥45 μg/dL1 BLL (μg/dL) Agent, Dosage,* and Administration Special Considerations Follow-up <45 Chelation therapy not routinely recommended See Reverse for Recommended Follow-up Blood Lead Test Schedule for Children 45 to <70 • DMSA (succimer, 2,3-meso-dimercaptosuccinic acid) • Monitor for anemia, • Schedule weekly health care visits • 1050 mg DMSA / m2 / 24 hours* ÷ q8 hours PO x neutropenia, and to monitor compliance and signs of toxicity. 5 days; round dose to nearest 100 mg/day, and then hepatic toxicity. • Monitor BLLs weekly until level stabilizes, ÷ 100-mg capsules as evenly as possible for q8-hour then follow Recommended Follow-up dosing schedule. -

Effect of Chromium(VI) on Serum Iron and Removal of Its Toxicity by Combining Deferasirox and Deferiprone Chelators in Rats
American Journal of Pharmacology and Toxicology 8 (4): 164-169, 2013 ISSN: 1557-4962 ©2013 Science Publication doi:10.3844/ajptsp.2013.164.169 Published Online 8 (4) 2013 (http://www.thescipub.com/ajpt.toc) Effect of Chromium(VI) on Serum Iron and Removal of its Toxicity by Combining Deferasirox and Deferiprone Chelators in Rats 1S. Jamil A. Fatemi, 1Marzieh Iranmanesh and 2Faezeh Dahooee Balooch 1Department of Chemistry, Faculty of Sciences, Islamic Azad University, Kerman Branch, Kerman, Iran 2Department of Chemistry, Faculty of Sciences, Shahid Bahonar University of Kerman, Kerman, Iran Received 2013-09-26, Revised 2013-10-21; Accepted 2013-10-30 ABSTRACT The present research is aimed to characterize the potential efficiency of two chelators after chromium(VI) administration for 60 days following two doses of 15 and 30 mg kg −1 chromium(VI) per body weight daily to male rats. However, the hypothesis that the two chelators might be more efficient as combined therapy than as single therapy in removing chromium(VI) from bood serum was considered. In this way, two known chelators deferasirox and deferiprone were chosen and tested in the acute rat model. Two chelators were given orally as a single or combined therapy for a period of one week. Chromium(VI) and iron concentrations in blood were determined by flame atomic absorption spectroscopy method. Chromium is one of the most widely used industrial metals. Several million workers worldwide are estimated to be exposed to chromium compounds in an array of industries. Chromium(VI) is more readily absorbed by both inhalation and oral routes. Ingestion of large amounts of chromium(VI) can lead to severe respiratory, cardiovascular, gastrointestinal, hepatic and renal damage and potentially death. -

AX Pharmaceutical Corp Product List
AX Pharmaceutical Corp Product List A 4-Aminopyridine Acetazolamine ACTH 1-24 Acyclovir Sodium Adenosine 5 Monophosphate Adenosine 5 Tri-Phosphate Disodium Salt Albendazole Alendronate Sodium USP Alternogest Aminopentamide Sulfate Aminophylline Amlodipine Besylate Amoxicillin/Clavulanate Potassium 4:1 Amoxicillin Trihydrate Amphotericin B Ampicillin Anastrozole Aripepazole Aripiprazole Atipamezole HCl Atovaquone USP Atropine Sulfate Monohydrate Avanafil Azelastine HCl B Baclofen Benazepril HCl Betahistine Dihydrochloride Betaine Hydrochloride Betamethasone Acetate Betamethasone Dipropionate Betamethasone Sodium Phosphate Betaxolol Bexarotene Bicalutamide Bimatoprost Bisacodyl Bismuth Subcarbonate Bismuth Subsalicylate Bleomycin A5 Hydrochloride Bleomycin Sulfate Bretylium Tosylate Brimonidine Brinzolamide Bromhexine Bromocriptine Mesylate Brompheniramine Maleate Budesonide Bumetanide Bupivacaine Base Bupivacaine Hydrochloride Buprenorphine Bupropion HCl Buspirone Hydrochloride Busulfan Butaphosphan Butylated Hydroxyanisole C Cabergoline Calamine Calcium Glycerophosphate Calcium Levulinate Dihydrate Capsaicin Captopril Carbamazepine Carbazochrome Carbenoxolone Carbetocin Acetate Carbidopa Carbocisteine Carboplatin Carmustine Carprofen Carvedilol Cefadroxil Hemihydrate Cefadroxil Monohydrate Cefazolin Cefazolin Sodium Cefdinir Cefotaxime Cefotetan Disodium Cefpodoxime Cefpodoxime Proxetil Ceftazidime Ceftiofur Free Acid Ceftiofur Sodium Ceftriaxone Cefuroxime (Ceftin) Celecoxib Cephalexin Base Cephalexin Monohydrate Cesium Chloride Cetirizine -

Treatment Strategies in Alzheimer's Disease: a Review with Focus On
Biometals (2016) 29:827–839 DOI 10.1007/s10534-016-9959-8 Treatment strategies in Alzheimer’s disease: a review with focus on selenium supplementation Jan Aaseth . Jan Alexander . Geir Bjørklund . Knut Hestad . Petr Dusek . Per M. Roos . Urban Alehagen Received: 24 July 2016 / Accepted: 25 July 2016 / Published online: 16 August 2016 Ó The Author(s) 2016. This article is published with open access at Springerlink.com Abstract Alzheimer’s disease (AD) is a neurode- inhibition of enzymes responsible for its formation, or generative disorder presenting one of the biggest to promote resolution of existing cerebral Ab plaques. healthcare challenges in developed countries. No However, these approaches have failed to demonstrate effective treatment exists. In recent years the main significant cognitive improvements. Intracellular focus of AD research has been on the amyloid rather than extracellular events may be fundamental hypothesis, which postulates that extracellular precip- in AD pathogenesis. Selenate is a potent inhibitor of itates of beta amyloid (Ab) derived from amyloid tau hyperphosphorylation, a critical step in the precursor protein (APP) are responsible for the formation of neurofibrillary tangles. Some selenium cognitive impairment seen in AD. Treatment (Se) compounds e.g. selenoprotein P also appear to strategies have been to reduce Ab production through protect APP against excessive copper and iron J. Aaseth Á K. Hestad P. M. Roos (&) Department of Research, Innlandet Hospital Trust, Institute of Environmental Medicine, IMM, Karolinska Brumunddal, Norway Institutet, Nobels va¨g 13, Box 210, 17177 Stockholm, Sweden J. Aaseth Á K. Hestad e-mail: [email protected] Department of Public Health, Hedmark University of Applied Sciences, Elverum, Norway P. -

The Regional Center for Poison Control and Prevention Serving Massachusetts and Rhode Island
The Regional Center for Poison Control and Prevention Serving Massachusetts and Rhode Island Poison Potential Antidote Acetaminophen n-Acetylcysteine [Mucomyst®] Anticholinergics Physostigmine [Antilirium®] Benzodiazepines Flumazenil [Romazicon®] Beta-adrenergic blockers Glucagon Botulinum toxin Trivalent ABE botulinum antitoxin Calcium chloride or calcium gluconate Calcium channel blockers Hyperinsulinemia-euglycemia (HIE) therapy Atropine Carbamates Pralidoxime (2-PAM) [Protopam®] Carbon monoxide Oxygen; Hyperbaric oxygen (HBO) Clonidine Naloxone [Narcan®] Cyanide Cyanide Kit (Amyl/sodium nitrite, sodium thiosulfate) Digoxin (Cardiac glycosides) Digoxin Immune FAB Ovine [Digibind®, Digifab®] Epi Pen (Epinephrine SQ) Phentolamine [Regitine®] Ethanol Ethylene glycol 4-Methylpyrazole (Fomepizole) [Antizol®] Fluoride Calcium chloride or calcium gluconate Heparins Protamine Hydrofluoric acid Calcium chloride or calcium gluconate Hydrogen sulfide Oxygen; Hyperbaric oxygen (HBO); Sodium nitrite Iodine Starch Isoniazid Pyridoxine (Vitamin B6 ) METALS Dimercaprol [BAL] Arsenic Dimercaptosuccinic acid (DMSA, succimer) [Chemet®] Bismuth Dimercaprol [BAL] Copper D-Penicillamine [Cuprimine®] Gold Dimercaprol [BAL] Iron Deferoxamine [Desferal®] Dimercaprol [BAL] Edetate calcium disodium (Calcium EDTA) Lead Dimercaptosuccinic acid (DMSA, succimer) [Chemet®] D-Penicillamine [Cuprimine®] Dimercaprol [BAL] Mercury Dimercaptosuccinic acid (DMSA, succimer) [Chemet®] Ethanol Methanol 4-Methylpyrazole (Fomepizole) [Antizol®] Methemoglobinemic agents Methylene -

Molecular Targets of Manganese-Induced Neurotoxicity: a Five-Year Update
International Journal of Molecular Sciences Review Molecular Targets of Manganese-Induced Neurotoxicity: A Five-Year Update Alexey A. Tinkov 1,2, Monica M. B. Paoliello 3,4, Aksana N. Mazilina 5, Anatoly V. Skalny 6,7, Airton C. Martins 3 , Olga N. Voskresenskaya 2 , Jan Aaseth 2,8 , Abel Santamaria 9, Svetlana V. Notova 10,11, Aristides Tsatsakis 2,12 , Eunsook Lee 13 , Aaron B. Bowman 14 and Michael Aschner 2,3,* 1 Laboratory of Ecobiomonitoring and Quality Control, Yaroslavl State University, 150003 Yaroslavl, Russia; [email protected] 2 Laboratory of Molecular Dietetics, Department of Neurological Diseases and Neurosurgery, Department of Analytical and Forensic Toxicology, IM Sechenov First Moscow State Medical University (Sechenov University), 119435 Moscow, Russia; [email protected] (O.N.V.); [email protected] (J.A.); [email protected] (A.T.) 3 Department of Molecular Pharmacology, Albert Einstein College of Medicine, Bronx, NY 10461, USA; [email protected] (M.M.B.P.); [email protected] (A.C.M.) 4 Graduate Program in Public Health, Center of Health Sciences, State University of Londrina, Londrina, PR 86038-350, Brazil 5 Department of Medical Elementology, Peoples’ Friendship University of Russia (RUDN University), 117198 Moscow, Russia; [email protected] 6 World-Class Research Center “Digital Biodesign and Personalized Healthcare”, IM Sechenov First Moscow State Medical University (Sechenov University), 119435 Moscow, Russia; [email protected] 7 Laboratory of Medical Elementology, -

HANDBOOKS Published
HANDBOOKS published: "Handbook on Toxicity of Inorganic Compounds" (ISBN: 0-8247-7727-1) edited by Hans G. Seiler, Helmut Sigel, and Astrid Sigel; Marcel Dekker, Inc.; New York, Basel; 1st published 1988; 3rd printing; 1069 pages 1. Scope and Use of the Handbook 14. Cadmium Helmut Sigel and Hans G. Seiler June K. Dunnick and Bruce A. Fowler 2. Bioinorganic Chemistry of Toxicity 15. Calcium R. Bruce Martin Nicholas J. Birch 3. General Aspects of Toxicology 16. Carbon John Savory, Roger L. Bertholf, and Hans R. Zorn, Werner F. Diller, Rainer Michael R. Wills Eisenmann, Klaus J. Freundt, Klaus D. Friedberg, Klaus Mengel, Rainer Schiele, 4. Some Recommendations for the Specimen and Gerhard Triebig Collection of Biological Materials for Analysis 17. Cesium Hans G. Seiler Robert J. Davie and Iain P. L. Coleman 5. Actinium 18. Chlorine Werner Burkart Ulrich Ewers, Nicolai Manojlovic, Wolfgang Hadnagy, and Yash Paul 6. Aluminum Grover Roger L. Bertholf, Michael R. Wills, and John Savory 19. Chromium Joshua W. Hamilton and 7. Antimony Karen E. Wetterhahn Rolf Iffland 20. Cobalt 8. Arsenic Jürgen Angerer and Regine Heinrich Wolfgang Arnold 21. Copper 9. Barium Bibudhendra Sarkar Gottfried Machata 22. Fluorine 10. Beryllium Guido Sticht Hans R. Zorn, Thomas W. Stiefel, Jörg Beuers, and Ronald Schlegelmilch 23. Gallium Raymond L. Hayes 11. Bismuth 24. Germanium David W. Thomas, T. F. Hartley, G. B. Gerber P. Coyle, and S. Sobecki 25. Gold 12. Boron Blaine M. Sutton and Lynn A. Larsen Michael J. DiMartino 13. Bromine 26. Hafnium Guido Sticht and Herbert Käferstein Martine Duverger-van Bogaert and Marie Lambotte-Vandepaer 26.