Reproduction Biology and Embryonic Development PAUL JIVOFF, ANSON H
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lady Crabs, Ovalipes Ocellatus, in the Gulf of Maine
18_04049_CRABnotes.qxd 6/5/07 8:16 PM Page 106 Notes Lady Crabs, Ovalipes ocellatus, in the Gulf of Maine J. C. A. BURCHSTED1 and FRED BURCHSTED2 1 Department of Biology, Salem State College, Salem, Massachusetts 01970 USA 2 Research Services, Widener Library, Harvard University, Cambridge, Massachusetts 02138 USA Burchsted, J. C. A., and Fred Burchsted. 2006. Lady Crabs, Ovalipes ocellatus, in the Gulf of Maine. Canadian Field-Naturalist 120(1): 106-108. The Lady Crab (Ovalipes ocellatus), mainly found south of Cape Cod and in the southern Gulf of St. Lawrence, is reported from an ocean beach on the north shore of Massachusetts Bay (42°28'60"N, 70°46'20"W) in the Gulf of Maine. All previ- ously known Gulf of Maine populations north of Cape Cod Bay are estuarine and thought to be relicts of a continuous range during the Hypsithermal. The population reported here is likely a recent local habitat expansion. Key Words: Lady Crab, Ovalipes ocellatus, Gulf of Maine, distribution. The Lady Crab (Ovalipes ocellatus) is a common flats (Larsen and Doggett 1991). Lady Crabs were member of the sand beach fauna south of Cape Cod. not found in intensive local studies of western Cape Like many other members of the Virginian faunal Cod Bay (Davis and McGrath 1984) or Ipswich Bay province (between Cape Cod and Cape Hatteras), it (Dexter 1944). has a disjunct population in the southern Gulf of St. Berrick (1986) reports Lady Crabs as common on Lawrence (Ganong 1890). The Lady Crab is of consid- Cape Cod Bay sand flats (which commonly reach 20°C erable ecological importance as a consumer of mac- in summer). -

Stomach Content Analysis of Cobia, Rachycentron Canadum, from Lower
665 Stomach content analysis of cobia, movement of cobia within lower Chesa peake Bay during summer, as well as Rachycentron canadum, the return of individual cobia to spe from lower Chesapeake Bay* cific locations or general regions of the lower Bay in subsequent summers.1 Al though Chesapeake Bay is an impor Michael D. Arendt tant destination for migrating cobia, School of Marine Science feeding habits of cobia in the Bay have College of William and Mary never been thoroughly examined. Our Virginia Institute of Marine Science study documents cobia feeding habits Gloucester Point, Virginia 23062 in Chesapeake Bay and compares find Present address: Marine Resources Research Institute ings with similar cobia studies from South Carolina Department of Natural Resources Division North Carolina and the northern Gulf 217 Fort Johnson Road Charleston, South Carolina 29422-2559 of Mexico. E-mail address: [email protected] Methods John E. Olney Department of Fisheries Science Cobia were sampled opportunistically School of Marine Science at marinas and fishing tournaments College of William and Mary in lower Chesapeake Bay between Virginia Institute of Marine science June and July 1997. Intact stomachs Gloucester Point, Virginia 23062 were removed by cutting above the car diac sphincter (esophagus) and below Jon A. Lucy the pyloric sphincter (large intestine). Stomachs were labeled, bagged, trans Sea Grant Marine Advisory Program Virginia Institute of Marine Science ported on ice to the VA Institute of Glooucester Point, Virginia 23062 Marine Science, and examined in rela tively fresh condition. An incision was made along the longitudinal axis and the contents of stomachs were emp tied onto a 500-µm mesh sieve for rins ing and sorting. -

A Review of Blue Crab Predators Status: TAES San Antonio Phone: 830-214-5878 Note: E-Mail: [email protected]
********************************************************************* ********************************************************************* Document-ID: 2225347 Patron: Note: NOTICE: ********************************************************************* ********************************************************************* Pages: 16 Printed: 02-22-12 11:45:34 Sender: Ariel/Windows Journal Title: proceedings of the blue crab 2/22/2012 8:35 AM , mortality symposium (ult state marine fisheries (Please update within 24 hours) commission publication) Ceil! #: SH380.45. L8 858 1999 Volume: Issue: 90 Month/Year: Pages: Nof Wanted 08/19/2012 Da~e: l' Article Author: Guillory, V and M Elliot Article Title: A review of blue crab predators Status: TAES San Antonio Phone: 830-214-5878 Note: E-mail: [email protected] Name: Bandel, Micaela T AES San Antonio 2632 Broadway, Suite 301 South San Antonio, TX 78215 I '' I i' Proceedings ofthe Blue Crab Symposium 69-83 n of d A Review of Blue Crab Predators \n. I ~s VINCENT GUILLORY AND MEGAN ELLIOT B- Louisiana Department of Wildlife and Fisheries, P.O. Box 189, Bourg, Lou,,fana 70343 Abstract. - The diverse life history stages, abundance, and wide distributio> over a variety of habitats are attributes that expose blue crabs (Callinectes sapidus Rathbun) to nuinerous predators. An extensive literature search was undertaken on food habits of marine and estuarin,· invertebrate, and vertebrate species to identify predators of blue crab zoea, megalopae, and juveni k/adults. Ninety three species, which included invertebrates, fish, reptiles, birds, and mammals, were documented to prey upon blue crabs. An additional l l 9 sp~cies had other crab species or brachyur:m remains in their stomach contents. More fish species were identified as blue crab predators than any other taxonomic group (67), and 60 fish species were documented to prey upon unidentified crabs and/or brachyurans. -

Needham Notes
Lesson 26 Lesson Outline: Exercise #1 - Basic Functions Exercise #2 - Phylogenetic Trends Exercise #3 - Case Studies to Compare • Reproductive Strategies- Energy Partitioning • External versus Internal Fertilization • Sexual Dimorphism o Functional Characteristics o Aids to Identification o Copulatory Organs • Timing - Copulation, Ovulation, Fertilization, Development Objectives: Throughout the course what you need to master is an understanding of: 1) the form and function of structures, 2) the phylogenetic and ontogenetic origins of structures, and 3) the extend to which various structures are homologous, analogous and/or homoplastic. At the end of this lesson you should be able to: Describe the advantages and disadvantages of internal and external fertilization Describe sexual dimorphism and the selection pressures that lead to it Describe the trends seen in the design of copulatory organs Describe the various forms of reproductive strategy for delaying development of the fertilized egg and the selective advantage of them References: Chapter 15: 351-386 Reading for Next Lesson: Chapter 16: 387- 428 Exercise #1 List the basic functions of the urogenital system: The urinary system excretes the waste products of cellular digestion, ions, amino acids, salts, etc. It also plays a key role in water balance along with numerous other structures in different species living in different environments (i.e. gills, skin, salt glands). The primary function of the system is to give rise to offspring, - to reproduce. Exercise #2 Describe the evolutionary trends that we see in the urogenital systems of the different vertebrate groups: The phylogenetic trends that we see throughout the chordates were covered in detail in lectures (lecture 31 and 32) and are summarized schematically in the next figures: Exercise #3 – Comparisons – Case 1 Reproductive Strategies - Energy Partitioning Some would argue that the primary reason that organisms exist is to reproduce and make more organisms. -

Lady Crab, Occurs Along in Nova Scotia They Occur in Several Other Coastal Areas the Eastern Seaboard of Including the Bay of Fundy and Minas Basin
Moulted shells are found at Burntcoat Head. Ovalipes ocellatus Class: Malacostraca Order: Decapoda Family: Portunidae Genus: Ovalipes Distribution Crabs in the genus ovalipes In Canada there are disjunct populations in the Gulf of St. are distributed worldwide. Lawrence and the Northumberland Strait. The Northumberland This specific species, Strait is a tidal water body between Prince Edward Island and Ovalipes ocellatus, known as the coast of eastern New Brunswick and northern Nova Scotia. the lady crab, occurs along In Nova Scotia they occur in several other coastal areas the eastern seaboard of including the Bay of Fundy and Minas Basin. Most populations North America, from occur south of Cape Cod, Massachusetts and continue south Canada south to Florida. along the Atlantic coast. They converge with two other species of Ovalipes; O. stephenson and O. floridanus. Habitat They live in shallow coastal It is most often found in sandy substrates in quite shallow water, waters and off shore areas including mud banks sand bars. It may occur in surf zones with sandy bottoms. where there is strong wave action and constantly shifting sands. Food The lady crab feeds on live or decaying marine organisms such It is both a predator and a as fish, crabs, or clams. When fish or worms pass by, it comes scavenger. out of the sand and grabs the animal with its claws. Reproduction Courtship takes place The testes or ovaries are situated dorsally in the thorax. Testes between these crabs with open externally in the male near the basal segment of the last the male following and then pair of legs while the ovaries in the female open externally on holding on to a female. -

Food of the Red Drum, Sciaenops Ocellata, from Mississippi Sound
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Faculty Publications from the Harold W. Manter Laboratory of Parasitology Parasitology, Harold W. Manter Laboratory of 1978 Food of the Red Drum, Sciaenops ocellata, from Mississippi Sound Robin M. Overstreet Gulf Coast Research Laboratory, [email protected] Richard W. Heard University of Southern Mississippi, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/parasitologyfacpubs Part of the Parasitology Commons Overstreet, Robin M. and Heard, Richard W., "Food of the Red Drum, Sciaenops ocellata, from Mississippi Sound" (1978). Faculty Publications from the Harold W. Manter Laboratory of Parasitology. 488. https://digitalcommons.unl.edu/parasitologyfacpubs/488 This Article is brought to you for free and open access by the Parasitology, Harold W. Manter Laboratory of at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Faculty Publications from the Harold W. Manter Laboratory of Parasitology by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. GulfResearch Reports, Vol. 6, No. 2, 131-135, 1978 FOOD OF THE RED DRUM, SCIAENOPS OCELLATA, FROM MISSISSIPPI SOUND’ ROBIN M. OVERSTREET AND RICHARD W. HEARD Parasitology Section, Gurf Coast Research Laboratory, Ocean Springs, Mississippi 39564 ABSTRACT Examined digestive tracts of the red drum in Mississippi Sound contained mostly decapod crustaceans. Crustaceans accounted for 34 of 59 encountered taxa, more than reported from any other region. Nevertheless, the general diet for 104 fish with food contents out of the 107 examined is similar to that reported for red drum in several other studies from other areas. In addition to crustaceans, fishes followed by polychaetes occurred as the most important items (in 99, 43, and 15% of the drum with food, respectively). -

A Biological Switching Valve Evolved in the Female of a Sex-Role Reversed
RESEARCH ARTICLE A biological switching valve evolved in the female of a sex-role reversed cave insect to receive multiple seminal packages Kazunori Yoshizawa1*, Yoshitaka Kamimura2, Charles Lienhard3, Rodrigo L Ferreira4, Alexander Blanke5,6 1Laboratory of Systematic Entomology, School of Agriculture, Hokkaido University, Sapporo, Japan; 2Department of Biology, Keio University, Yokohama, Japan; 3Natural History Museum of Geneva, Geneva, Switzerland; 4Biology Department, Federal University of Lavras, Lavras, Brazil; 5Institute for Zoology, University of Cologne, Zu¨ lpicher, Ko¨ ln; 6Medical and Biological Engineering Research Group, School of Engineering and Computer Science, University of Hull, Hull, United Kingdom Abstract We report a functional switching valve within the female genitalia of the Brazilian cave insect Neotrogla. The valve complex is composed of two plate-like sclerites, a closure element, and in-and-outflow canals. Females have a penis-like intromittent organ to coercively anchor males and obtain voluminous semen. The semen is packed in a capsule, whose formation is initiated by seminal injection. It is not only used for fertilization but also consumed by the female as nutrition. The valve complex has two slots for insemination so that Neotrogla can continue mating while the first slot is occupied. In conjunction with the female penis, this switching valve is a morphological novelty enabling females to compete for seminal gifts in their nutrient-poor cave habitats through long copulation times and multiple seminal injections. The evolution of this switching valve may *For correspondence: have been a prerequisite for the reversal of the intromittent organ in Neotrogla. [email protected] DOI: https://doi.org/10.7554/eLife.39563.001 Competing interests: The authors declare that no competing interests exist. -

Shrimps, Lobsters, and Crabs of the Atlantic Coast of the Eastern United States, Maine to Florida
SHRIMPS, LOBSTERS, AND CRABS OF THE ATLANTIC COAST OF THE EASTERN UNITED STATES, MAINE TO FLORIDA AUSTIN B.WILLIAMS SMITHSONIAN INSTITUTION PRESS Washington, D.C. 1984 © 1984 Smithsonian Institution. All rights reserved. Printed in the United States Library of Congress Cataloging in Publication Data Williams, Austin B. Shrimps, lobsters, and crabs of the Atlantic coast of the Eastern United States, Maine to Florida. Rev. ed. of: Marine decapod crustaceans of the Carolinas. 1965. Bibliography: p. Includes index. Supt. of Docs, no.: SI 18:2:SL8 1. Decapoda (Crustacea)—Atlantic Coast (U.S.) 2. Crustacea—Atlantic Coast (U.S.) I. Title. QL444.M33W54 1984 595.3'840974 83-600095 ISBN 0-87474-960-3 Editor: Donald C. Fisher Contents Introduction 1 History 1 Classification 2 Zoogeographic Considerations 3 Species Accounts 5 Materials Studied 8 Measurements 8 Glossary 8 Systematic and Ecological Discussion 12 Order Decapoda , 12 Key to Suborders, Infraorders, Sections, Superfamilies and Families 13 Suborder Dendrobranchiata 17 Infraorder Penaeidea 17 Superfamily Penaeoidea 17 Family Solenoceridae 17 Genus Mesopenaeiis 18 Solenocera 19 Family Penaeidae 22 Genus Penaeus 22 Metapenaeopsis 36 Parapenaeus 37 Trachypenaeus 38 Xiphopenaeus 41 Family Sicyoniidae 42 Genus Sicyonia 43 Superfamily Sergestoidea 50 Family Sergestidae 50 Genus Acetes 50 Family Luciferidae 52 Genus Lucifer 52 Suborder Pleocyemata 54 Infraorder Stenopodidea 54 Family Stenopodidae 54 Genus Stenopus 54 Infraorder Caridea 57 Superfamily Pasiphaeoidea 57 Family Pasiphaeidae 57 Genus -

Feeding Habits of the Tiger Shark, Galeocerdo Cuvier, in the Northwest Atlantic Ocean and Gulf of Mexico
FEEDING HABITS OF THE TIGER SHARK, GALEOCERDO CUVIER, IN THE NORTHWEST ATLANTIC OCEAN AND GULF OF MEXICO by Alexandra Aines Dr. Andre Boustany, Advisor Dr. Pat Halpin, Advisor April 28, 2017 Masters project submitted in partial fulfillment of the requirements for the Master of Environmental Management degree in the Nicholas School of the Environment of Duke University EXECUTIVE SUMMARY Sharks are apex predators that structure marine communities through predation. Studies of diet and feeding patterns contribute to developing ecosystem models and it is critical that whenever possible, diet information specific to the area of concern is applied when developing an ecosystem model. Despite a number of studies conducted on tiger shark feeding, most comprehensive studies have been from the Pacific Ocean. There is little knowledge of the diet of tiger sharks in the Atlantic Ocean and Gulf of Mexico, with most studies in that area being localized and anecdotal. This study examines diet by life stage and tests for the presence of ontogenetic shifts, as well as how the diet of these sharks might be changing under certain environmental conditions and geographic variations. This study reviews the information collected on tiger shark feeding to date. It summarizes the results from previous studies on tiger shark diet: what prey items were found in studies of Pacific tiger sharks, and results from the anecdotal studies in the Atlantic, most of which took place in the early to mid 1900s, included small sample sizes, and were conducted in localized regions. For this study 169 sharks were caught as part of the U.S. -

Invertebrate ID Guide
11/13/13 1 This book is a compilation of identification resources for invertebrates found in stomach samples. By no means is it a complete list of all possible prey types. It is simply what has been found in past ChesMMAP and NEAMAP diet studies. A copy of this document is stored in both the ChesMMAP and NEAMAP lab network drives in a folder called ID Guides, along with other useful identification keys, articles, documents, and photos. If you want to see a larger version of any of the images in this document you can simply open the file and zoom in on the picture, or you can open the original file for the photo by navigating to the appropriate subfolder within the Fisheries Gut Lab folder. Other useful links for identification: Isopods http://www.19thcenturyscience.org/HMSC/HMSC-Reports/Zool-33/htm/doc.html http://www.19thcenturyscience.org/HMSC/HMSC-Reports/Zool-48/htm/doc.html Polychaetes http://web.vims.edu/bio/benthic/polychaete.html http://www.19thcenturyscience.org/HMSC/HMSC-Reports/Zool-34/htm/doc.html Cephalopods http://www.19thcenturyscience.org/HMSC/HMSC-Reports/Zool-44/htm/doc.html Amphipods http://www.19thcenturyscience.org/HMSC/HMSC-Reports/Zool-67/htm/doc.html Molluscs http://www.oceanica.cofc.edu/shellguide/ http://www.jaxshells.org/slife4.htm Bivalves http://www.jaxshells.org/atlanticb.htm Gastropods http://www.jaxshells.org/atlantic.htm Crustaceans http://www.jaxshells.org/slifex26.htm Echinoderms http://www.jaxshells.org/eich26.htm 2 PROTOZOA (FORAMINIFERA) ................................................................................................................................ 4 PORIFERA (SPONGES) ............................................................................................................................................... 4 CNIDARIA (JELLYFISHES, HYDROIDS, SEA ANEMONES) ............................................................................... 4 CTENOPHORA (COMB JELLIES)............................................................................................................................ -
St. Lucie, Units 1 and 2
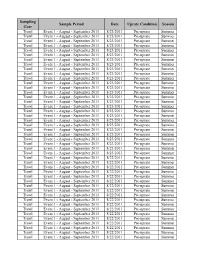
Sampling Sample Period Date Uprate Condition Season Gear Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event 1 - August - September 2011 8/23/2011 Pre-uprate Summer Trawl Event -

Reproductive System Further Information: Fish Reproduction and Spawn (Biology)
Reproductive system Further information: Fish reproduction and Spawn (biology) Organs: 1. Liver, 2. Gas bladder, 3. Roe, 4. Pyloric caeca, 5. Stomach, 6. Intestine Fish reproductive organs include testicles and ovaries. In most species, gonads are paired organs oF similar size, which can be partially or totally Fused.[52] There may also be a range oF secondary organs that increase reproductive Fitness. In terms oF spermatogonia distribution, the structure oF teleosts testes has two types: in the most common, spermatogonia occur all along the seminiFerous tubules, while in atherinomorph fish they are confined to the distal portion oF these structures. Fish can present cystic or semi- cystic spermatogenesis in relation to the release phase oF germ cells in cysts to the seminiFerous tubules lumen.[52] Fish ovaries may be oF three types: gymnovarian, secondary gymnovarian or cystovarian. In the First type, the oocytes are released directly into the coelomic cavity and then enter the ostium, then through the oviduct and are eliminated. Secondary gymnovarian ovaries shed ova into the coelom From which they go directly into the oviduct. In the third type, the oocytes are conveyed to the exterior through the oviduct.[53] Gymnovaries are the primitive condition Found in lungFish, sturgeon, and bowFin. Cystovaries characterize most teleosts, where the ovary lumen has continuity with the oviduct.[52] Secondary gymnovaries are Found in salmonids and a Few other teleosts. Oogonia development in teleosts Fish varies according to the group, and the determination oF oogenesis dynamics allows the understanding oF maturation and Fertilization processes. Changes in the nucleus, ooplasm, and the surrounding layers characterize the oocyte maturation process.[52] Postovulatory follicles are structures Formed aFter oocyte release; they do not have endocrine Function, present a wide irregular lumen, and are rapidly reabsorbed in a process involving the apoptosis oF Follicular cells.