Expression of Atrial and Brain Natriuretic Peptides and Their Genes in Hearts of Patients with Cardiac Amyloidosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Study of the Formation of Oligomers and Amyloid Plaques from Amylin by Capillary Electrophoresis and Fluorescent Microchip E
University of Arkansas, Fayetteville ScholarWorks@UARK Biological and Agricultural Engineering Biological and Agricultural Engineering Undergraduate Honors Theses 5-2015 The tuds y of the formation of oligomers and amyloid plaques from Amylin by capillary electrophoresis and fluorescent microchip electrophoresis Shane Weindel University of Arkansas, Fayetteville Follow this and additional works at: http://scholarworks.uark.edu/baeguht Part of the Engineering Commons Recommended Citation Weindel, Shane, "The tudys of the formation of oligomers and amyloid plaques from Amylin by capillary electrophoresis and fluorescent microchip electrophoresis" (2015). Biological and Agricultural Engineering Undergraduate Honors Theses. 25. http://scholarworks.uark.edu/baeguht/25 This Thesis is brought to you for free and open access by the Biological and Agricultural Engineering at ScholarWorks@UARK. It has been accepted for inclusion in Biological and Agricultural Engineering Undergraduate Honors Theses by an authorized administrator of ScholarWorks@UARK. For more information, please contact [email protected], [email protected]. The Study of the Formation of Oligomers and Amyloid Plaques from Amylin by Capillary Electrophoresis and Fluorescent Microchip Electrophoresis Shane Weindel, Biological Engineering Undergraduate Christa Hestekin, Chemical Engineering Assistant Professor Department of Biological & Agricultural Engineering 203 Engineering Hall 1 University of Arkansas Abstract Amylin, a pancreatic β-cell hormone, was the focus of this research project. This hormone is co-localized and co-secreted with insulin in response to nutrient stimuli. The hormone inhibits food intake, gastric emptying and glucagon secretion. Insulin and amylin appear to complement each other in the control of plasma glucose levels. Human amylin has a propensity to self-aggregate and to form insoluble bodies. -

Beta Amyloid Imaging with Positron Emission Tomography For
Corporate Medical Policy Beta Amyloid Imaging With Positron Emission Tomography for Alzheimer’s Disease File Name: beta_amyloid_imaging_with_positron_emission_tomography_for_alzheimers_disease Origination: 10/2014 Last CAP Review: 5/2021 Next CAP Review: 5/2022 Last Review: 5/2021 Description of Procedure or Service Three radioactive tracers (florbetapir F18, flutemetamol F18, florbetaben F18) that bind to beta amyloid and can be detected in vivo with positron emission tomography (PET) have been developed. This technology is being evaluated to detect beta amyloid neuritic plaque density in adult patients with cognitive impairment who are being evaluated for Alzheimer disease (AD) and/or other causes of cognitive decline. Background The diagnosis of AD is divided into 3 categories: possible, probable, and definite AD. A diagnosis of definite AD requires postmortem confirmation of AD pathology, including the presence of extracellular beta amyloid plaques and intraneuronal neurofibrillary tangles in the cerebral cortex. There can be a range of beta amyloid plaques and neurofibrillary tanges on histopathology that support a low, intermediate or high probability of AD. Probable AD dementia is diagnosed clinically when the patient meets core clinical criteria for dementia and has a typical clinical course for AD. A typical clinical course is defined as an insidious onset, with the initial and most prominent cognitive deficits being either amnestic or nonamnestic, e.g., language, visuospatial, or executive function deficits, and a history of progressively worsening cognition over time. A diagnosis of possible AD dementia is made when the patient meets the core clinical criteria for AD dementia but has an atypical course or an etiologically mixed presentation. Mild cognitive impairment (MCI) may be diagnosed when there is a change in cognition, but impairment is insufficient for the diagnosis of dementia. -

A Guide to Transthyretin Amyloidosis
A Guide to Transthyretin Amyloidosis Authored by Teresa Coelho, Bo-Goran Ericzon, Rodney Falk, Donna Grogan, Shu-ichi Ikeda, Mathew Maurer, Violaine Plante-Bordeneuve, Ole Suhr, Pedro Trigo 2016 Edition Edited by Merrill Benson, Mathew Maurer What is amyloidosis? Amyloidosis is a systemic disorder characterized by extra cellular deposition of a protein-derived material, known as amyloid, in multiple organs. Amyloidosis occurs when native or mutant poly- peptides misfold and aggregate as fibrils. The amyloid deposits cause local damage to the cells around which they are deposited leading to a variety of clinical symptoms. There are at least 23 different proteins associated with the amyloidoses. The most well-known type of amyloidosis is associated with a hematological disorder, in which amyloid fibrils are derived from monoclonal immunoglobulin light-chains (AL amyloidosis). This is associated with a clonal plasma cell disorder, closely related to and not uncommonly co-existing with multiple myeloma. Chronic inflammatory conditions such as rheumatoid arthritis or chronic infections such as bronchiectasis are associated with chronically elevated levels of the inflammatory protein, serum amyloid A, which may misfold and cause AA amyloidosis. The hereditary forms of amyloidosis are autosomal dominant diseases characterized by deposition of variant proteins, in dis- tinctive tissues. The most common hereditary form is transthyretin amyloidosis (ATTR) caused by the misfolding of protein monomers derived from the tetrameric protein transthyretin (TTR). Mutations in the gene for TTR frequently re- sult in instability of TTR and subsequent fibril formation. Closely related is wild-type TTR in which the native TTR protein, particu- larly in the elderly, can destabilize and re-aggregate causing non- familial cases of TTR amyloidosis. -

Cerebral Amyloidosis, Amyloid Angiopathy, and Their Relationship to Stroke and Dementia
65 Cerebral amyloidosis, amyloid angiopathy, and their relationship to stroke and dementia ∗ Jorge Ghiso and Blas Frangione β-pleated sheet structure, the conformation responsi- Department of Pathology, New York University School ble for their physicochemical properties and tinctoreal of Medicine, New York, NY, USA characteristics. So far, 20 different proteins have been identified as subunits of amyloid fibrils [56,57,60 (for review and nomenclature)]. Although collectively they Cerebral amyloid angiopathy (CAA) is the common term are products of normal genes, several amyloid precur- used to define the deposition of amyloid in the walls of sors contain abnormal amino acid substitutions that can medium- and small-size leptomeningeal and cortical arteries, arterioles and, less frequently, capillaries and veins. CAA impose an unusual potential for self-aggregation. In- is an important cause of cerebral hemorrhages although it creased levels of amyloid precursors, either in the cir- may also lead to ischemic infarction and dementia. It is a culation or locally at sites of deposition, are usually the feature commonly associated with normal aging, Alzheimer result of overexpression, defective clearance, or both. disease (AD), Down syndrome (DS), and Sporadic Cerebral Of all the amyloid proteins identified, less than half are Amyloid Angiopathy. Familial conditions in which amyloid known to cause amyloid deposition in the central ner- is chiefly deposited as CAA include hereditary cerebral hem- vous system (CNS), which in turn results in cognitive orrhage with amyloidosis of Icelandic type (HCHWA-I), fa- decline, dementia, stroke, cerebellar and extrapyrami- milial CAA related to Aβ variants, including hereditary cere- dal signs, or a combination of them. -
Recognizing TTR-FAP Transthyretin Familial Amyloid Polyneuropathy
This version is Global RC approved and local adaptation and approval is mandatory before distribution. Countries are responsible for language accuracy. Content to be updated with local information and labeling as required. Recognizing TTR-FAP Transthyretin Familial Amyloid Polyneuropathy About TTR-FAP TTR-FAP is a rare, genetic, TTR-FAP is caused by a mutation in the transthyretin gene, which can result in abnormal and unstable progressive and fatal transthyretin proteins. 2,3 neurodegenerative disease affecting an estimated 10,000 people worldwide.1 TTR-FAP – A Disease of Protein Misfolding Free Tetramer Folded Misfolded Toxic Intermediates Monamer Monamer & Amyloid Fibrils TTR-FAP affects men and women equally. Monamer Aggregation Misfolding Functional TTR Structures TTR Structures Symptoms usually begin to affect Associated with Pathology people in their 30s. These abnormal proteins build up and form toxic This varies with genetics and structures called amyloid fibrils, which may deposit in the peripheral nervous system, leading to a decline in ethnic background.2,3 The life neurologic function, or in other parts of the body, such expectancy for someone who as the heart, digestive system, and kidneys.2,3,4,5,6,7 is diagnosed with TTR-FAP is said to be about 10 years.13 Where is TTR-FAP Most Prevalent? There are clusters of TTR-FAP patients in Portugal, Japan, and Sweden.8 TTR-FAP is also found in countries such as the United States, various countries in Europe (e.g., France, Italy, Spain, Germany, and UK), Brazil, and Taiwan.9 Prevalence of TTR-FAP may vary by country of origin and by the type of TTR gene mutation.10 Symptoms of TTR-FAP Symptoms vary, but often, the feet Later, weakness gets worse in the and legs are affected first—with legs.11 The arms may be affected pain, tingling, numbness, or loss of too, starting at the figertips.11 the ability to feel hot and cold.11 Why Early Diagnosis is Key Although the disease affects people differently, it typically gets worse over time and can progress rapidly. -

Breakthroughs in Antemortem Diagnosis of Neurodegenerative Diseases COMMENTARY Glenn C
COMMENTARY Breakthroughs in antemortem diagnosis of neurodegenerative diseases COMMENTARY Glenn C. Tellinga,1 The World Health Organization forecasts that within 2 Abnormal cytoplasmic accumulation of a normally sol- decades neurodegenerative disorders will eclipse can- uble and unfolded protein called α-synuclein is the cer to become the foremost cause of death in the de- hallmark of diseases referred to as synucleinopathies. veloped world after cardiovascular disease. Accurate Neuronal deposition of α-synuclein aggregates in Lewy detection of pathological processes goes hand in hand bodies occurs in Parkinson’s disease (PD) and dementia with the goals of treatment and prevention and, in light with Lewy bodies (DLB). Yet another protein—the prion of their protracted but worsening clinical progression, protein (PrP)—is central to a group of interrelated dis- the earlier a diagnosis can be made the better. How- orders commonly referred to as prion diseases. ever, the challenge underlying accurate detection of Since the concept underlying the diagnostic ap- neurodegenerative diseases during their clinical phase proach taken by Metrick et al. (1) derives from studies is that specific biomarkers are not present at high of prions, it is worth reviewing what we have learned enough concentrations for routine detection in acces- about PrP and the applicability of these findings to sible specimens. Consequently, it has only been pos- other proteopathic diseases. The prion disorders are sible to definitively diagnose these conditions by transmissible neurodegenerative diseases affecting examination of brain pathology after death. The paper animals and humans. The most common human form by Metrick et al. (1) in PNAS addresses the issue of is Creutzfeldt–Jakob disease (CJD) which occurs most improved antemortem biomarker detection for a frequently as a sporadic, rapidly progressive condition spectrum of neurological disorders, using assays of older individuals. -

The Increasing Impact of Cerebral Amyloid Angiopathy: Essential New
JNNP Online First, published on August 26, 2017 as 10.1136/jnnp-2016-314697 Cerebrovascular disease J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp-2016-314697 on 26 August 2017. Downloaded from REVIEW The increasing impact of cerebral amyloid angiopathy: essential new insights for clinical practice Gargi Banerjee,1 Roxana Carare,2 Charlotte Cordonnier,3 Steven M Greenberg,4 Julie A Schneider,5 Eric E Smith,6 Mark van Buchem,7 Jeroen van der Grond,7 Marcel M Verbeek,8,9 David J Werring1 For numbered affiliations see ABSTRact Furthermore, CAA gained new relevance with the end of article. Cerebral amyloid angiopathy (CAA) has never been advent of anti-Aβ immunotherapies for the treat- more relevant. The last 5 years have seen a rapid ment of Alzheimer’s disease (AD), as a sizeable Correspondence to increase in publications and research in the field, with proportion of those treated went on to develop Dr David J Werring, The National Hospital for Neurology and the development of new biomarkers for the disease, imaging features of CAA-related inflammation as 5 Neurosurgery, UCL Institute thanks to advances in MRI, amyloid positron emission an unintended consequence. This, together with of Neurology, Queen Square, tomography and cerebrospinal fluid biomarker analysis. advances in our understanding of the impact of London WC1N 3BG, UK; d. The inadvertent development of CAA-like pathology CAA on cognition, in the context of ICH, ageing werring@ ucl. ac. uk in patients treated with amyloid-beta immunotherapy and AD, has broadened the clinical spectrum Received 1 March 2017 for Alzheimer’s disease has highlighted the importance of disease to which the contribution of CAA is Revised 26 April 2017 of establishing how and why CAA develops; without recognised. -

Laboratory Approach to the Diagnosis of Amyloidosis
Laboratory Approach to the Diagnosis of Amyloidosis Possible Patient Presentations ■ Nephrotic-range proteinuria with or without renal insuf ciency (nondiabetic) ■ In ltrative cardiomyopathy with restrictive hemodynamics and no ischemic history ■ Hepatomegaly with no lling defects visible by imaging ■ Nondiabetic peripheral neuropathy ■ Fatigue, weight loss, dyspnea, edema, or paresthesias ■ Macroglossia ■ Classic amyloid purpura ■ Atypical myeloma ALWAYS ORDER ■ PEISO / Protein Electrophoresis and Isotype, Serum ■ MPSU / Monoclonal Protein Study, 24 Hour, Urine ■ FLCS / Immunoglobulin Free Light Chains, Serum ■ Subcutaneous Fat Aspirate (fat aspirate with Congo red stain) Subcutaneous fat aspirate: Subcutaneous fat aspirate is negative but Congo red amyloid deposits are present clinician still has high index of suspicion Diagnostic for amyloidosis Specific organ biopsy with Congo red to assess for amyloid POSITIVE NEGATIVE Diagnostic for amyloidosis To identify the type of amyloid protein: Unlikely to be amyloidosis if involved organ biopsy Acceptable specimen is paraffin-embedded tissue, order: is negative. AMPIP / Amyloid Protein Identification, Paraffin, LC-MS/MS Special instructions applying ONLY to Mayo Clinic sites in Rochester, Jacksonville, and Scottsdale/Phoenix: If specimen is fat aspirate in RPMI media, order: Amyloid Protein Identification, Fat Aspirate, LC-MS/MS Prognostic/Monitoring Tests: ■ Echocardiography-Doppler-Strain ■ CRCL / Creatinine Clearance, Serum and 24-Hour Urine ■ TRPS / Troponin T, 5th Generation, Serum ■ PBNP / NT-Pro B-Type Natriuretic Peptide, Serum ■ FLCS / Immunoglobulin Free Light Chains, Serum ■ PCPRO / Plasma Cell DNA Content and Proliferation, Bone Marrow ■ MFCF / Myeloma, FISH, Fixed Cells Note: In cases of suspected familial amyloidosis, see Amyloidosis (Familial) Test Algorithm © Mayo Foundation for Medical Education and Research (MFMER). All rights reserved. 03/2021. -

From Brain to Heart: Possible Role of Amyloid-Β in Ischemic Heart Disease and Ischemia-Reperfusion Injury
International Journal of Molecular Sciences Review From Brain to Heart: Possible Role of Amyloid-β in Ischemic Heart Disease and Ischemia-Reperfusion Injury Giulia Gagno 1, Federico Ferro 1, Alessandra Lucia Fluca 1, Milijana Janjusevic 1, Maddalena Rossi 1, Gianfranco Sinagra 1, Antonio Paolo Beltrami 2 , Rita Moretti 3 and Aneta Aleksova 1,* 1 Cardiothoracovascular Department, Azienda Sanitaria Universitaria Giuliano Isontina (ASUGI) and University of Trieste, 34100 Trieste, Italy; [email protected] (G.G.); ff[email protected] (F.F.); alessandrafl[email protected] (A.L.F.); [email protected] (M.J.); [email protected] (M.R.); [email protected] (G.S.) 2 Department of Medicine (DAME), University of Udine, 33100 Udine, Italy; [email protected] 3 Department of Internal Medicine and Neurology, Neurological Clinic, 34100 Trieste, Italy; [email protected] * Correspondence: [email protected] or [email protected]; Tel.: +39-340-550-7762 Received: 3 December 2020; Accepted: 14 December 2020; Published: 17 December 2020 Abstract: Ischemic heart disease (IHD) is among the leading causes of death in developed countries. Its pathological origin is traced back to coronary atherosclerosis, a lipid-driven immuno-inflammatory disease of the arteries that leads to multifocal plaque development. The primary clinical manifestation of IHD is acute myocardial infarction (AMI),) whose prognosis is ameliorated with optimal timing of revascularization. Paradoxically, myocardium re-perfusion can be detrimental because of ischemia-reperfusion injury (IRI), an oxidative-driven process that damages other organs. Amyloid-β (Aβ) plays a physiological role in the central nervous system (CNS). Alterations in its synthesis, concentration and clearance have been connected to several pathologies, such as Alzheimer’s disease (AD) and cerebral amyloid angiopathy (CAA). -

AMYLOIDOSIS AWARENESS for Patients and Their Support Network, Including Physicians, Nurses and Medical Students
AMYLOIDOSIS AWARENESS For patients and their support network, including physicians, nurses and medical students Section Name Here 1 TABLE OF CONTENTS 1 One Minute Overview 1 2 What is Amyloidosis? 2 3 Types of Amyloidosis 7 4 Diagnosis 17 5 Treatments 26 6 Major Amyloidosis Centers 37 Published October 2013. 7 Online Resources 39 This booklet has been made with the guidance of Amyloidosis Support Groups. Special thanks to doctors Morie Gertz, Angela Dispenzieri, Martha Grogan, Shaji Kumar, Nelson Leung, Mathew Maurer, Maria Picken, Janice Wiesman, and Vaishali Sanchorawala. While the information herein is meant to be accurate, the medical sciences are ever advancing. As such, the content of this publication is presented for educational purposes only. It is not intended as medical advice. All decisions regarding medical care should be discussed with a qualified, practicing physician. Illustration artwork © Fairman Studios, LLC. Cover image: Amyloidosis often occurs in middle-age and older individuals, but also in patients in their 30s or 40s, and occasionally even younger. 1. ONE MINUTE OVERVIEW 2. WHAT IS AMYLOIDOSIS? All of the normal proteins in our body are biodegradable Throughout our lifetime, our DNA is coding for the manufac- and recyclable. Amyloidosis is a disease in which abnor- ture of small molecules called proteins. These proteins provide mal proteins (amyloid) are resistant to being broken down. the structure and function for nearly all of life’s biological pro- As a consequence, the amyloid proteins deposit and ac- cesses. Enzymes that facilitate our cells’ chemistry, hormones cumulate in the body’s tissues. If amyloid builds up in the that affect our body’s growth and regulation, and antibodies kidney, heart, liver, gastrointestinal tract or nerves, it causes that form our immune response are all examples of proteins in those organs to function poorly. -

Hereditary Cerebral Amyloid Angiopathy
Hereditary cerebral amyloid angiopathy Description Hereditary cerebral amyloid angiopathy is a condition that can cause a progressive loss of intellectual function (dementia), stroke, and other neurological problems starting in mid-adulthood. Due to neurological decline, this condition is typically fatal in one's sixties, although there is variation depending on the severity of the signs and symptoms. Most affected individuals die within a decade after signs and symptoms first appear, although some people with the disease have survived longer. There are many different types of hereditary cerebral amyloid angiopathy. The different types are distinguished by their genetic cause and the signs and symptoms that occur. The various types of hereditary cerebral amyloid angiopathy are named after the regions where they were first diagnosed. The Dutch type of hereditary cerebral amyloid angiopathy is the most common form. Stroke is frequently the first sign of the Dutch type and is fatal in about one third of people who have this condition. Survivors often develop dementia and have recurrent strokes. About half of individuals with the Dutch type who have one or more strokes will have recurrent seizures (epilepsy). People with the Flemish and Italian types of hereditary cerebral amyloid angiopathy are prone to recurrent strokes and dementia. Individuals with the Piedmont type may have one or more strokes and typically experience impaired movements, numbness or tingling (paresthesias), confusion, or dementia. The first sign of the Icelandic type of hereditary cerebral amyloid angiopathy is typically a stroke followed by dementia. Strokes associated with the Icelandic type usually occur earlier than the other types, with individuals typically experiencing their first stroke in their twenties or thirties. -
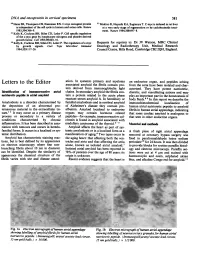
Identification of Immunoreactive Atrial Natriuretic Peptide in Atrial Amyloid
DNA and oncoprotein in cervical specimens 581 20Hann SR, Thompson CB, Eisenman RN. C-myc oncogene protein 23Makino R, Hayashi KA, Sugimura T. C-myc is induced in rat liver is independent of the cell cycle in human and avian cells. Nature at a very early stage of regeneration or by cycloheximide treat- 1985;314:366-9. ment. Nature 1984;310:697-8. Kelly K, Cochran BH, Stiles CD, Leder P. Cell specific regulation of the c-myc gene by lymphocyte mitogens and platelet derived growth factor. Cell 1983;35:603-10. Kelly K, Cochran BH, Stiles CD, Leder P. The regulation ofc-myc Requests for reprints to: Dr JV Watson, MRC Clinical by growth signals. Curr Tops Microbiol Immunol Oncology and Radiotherapy Unit, Medical Research 1984;133:1 17-26. Council Centre, Hills Road, Cambridge CB2 2QH, England. Letters to the Editor ation. In systemic primary and myeloma an endocrine organ, and peptides arising associated amyloid the fibrils contain pro- from the atria have been isolated and char- tein derived from immunoglobulin light acterised. They have potent natriuretic, Identification of imnunoreactive atrial chains. In secondary amyloid the fibrils con- diuretic, and vasodilating actions and may natriuretic peptide in atrial amyloid tain a protein related to the acute phase play an important part in the homeostasis of reactant serum amyloid A. In hereditary or body fluids.89 In this report we describe the Amyloidosis is a disorder characterised by familial amyloidosis and in cerebral amyloid immunohistochemical localisation of the deposition of an abnormal pro- of Alzheimer's disease they contain pre- human atrial natriuretic peptide to amyloid teinaceous material in the extracellular tis- albumin.