Point-Defect Chemistry on the Polarization Behavior of Niobium Doped Bismuth Titanate
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Open Cyphersthesis FINAL.Pdf
The Pennsylvania State University The Graduate School College of Engineering RESEARCH ON LOW FREQUENCY COMPOSITE TRANSDUCERS FABRICATED USING A SOL-GEL SPRAY-ON METHOD A Thesis in Engineering Science by Robert L. Cyphers c 2012 Robert L. Cyphers Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science December 2012 The thesis of Robert L. Cyphers was reviewed and approved∗ by the following: Bernhard R. Tittmann Schell Professor of Engineering Science and Mechanics Thesis Advisor Clifford Lissenden Professor of Engineering Science and Mechanics Mark W. Horn Professor of Engineering Science and Mechanics Judith A. Todd Professor of Engineering Science and Mechanics // P. B. Breneman Department Head Head of the Department of Engineering Science and Mechanics ∗Signatures are on file in the Graduate School. Abstract Ultrasonic nondestructive evaluation is currently used in countless applications to maintain a system's operational integrity. Piezoelectric transducers are the devices commonly used in this field to search for defects. A sol-gel fabrication method utilizing a spray-on deposition method has proven to produce ultrasonic transducers useful in harsh environments. This procedure produces thin film transducers, which adhere directly to a substrate making it favorable in use with irregular surface geometries. These transducers operate at relatively high frequencies due to their minute thickness. The objective of this research is to investigate the ability for low frequency operation into the low kilohertz range. Depositing thicker layers of piezoelectric composites, including bismuth titanate and lead zirconate titanate, led to adhesion problems between the metal substrates and ceramic material. Delamination of the piezoelectric elements was determined to be caused by a large mismatch in thermal expansion coefficients. -

High Temperature Ultrasonic Transducers: a Review
sensors Review High Temperature Ultrasonic Transducers: A Review Rymantas Kazys * and Vaida Vaskeliene Ultrasound Research Institute, Kaunas University of Technology, Barsausko st. 59, LT-51368 Kaunas, Lithuania; [email protected] * Correspondence: [email protected] Abstract: There are many fields such as online monitoring of manufacturing processes, non-destructive testing in nuclear plants, or corrosion rate monitoring techniques of steel pipes in which measure- ments must be performed at elevated temperatures. For that high temperature ultrasonic transducers are necessary. In the presented paper, a literature review on the main types of such transducers, piezoelectric materials, backings, and the bonding techniques of transducers elements suitable for high temperatures, is presented. In this review, the main focus is on ultrasonic transducers with piezoelectric elements suitable for operation at temperatures higher than of the most commercially available transducers, i.e., 150 ◦C. The main types of the ultrasonic transducers that are discussed are the transducers with thin protectors, which may serve as matching layers, transducers with high temperature delay lines, wedges, and waveguide type transducers. The piezoelectric materi- als suitable for high temperature applications such as aluminum nitride, lithium niobate, gallium orthophosphate, bismuth titanate, oxyborate crystals, lead metaniobate, and other piezoceramics are analyzed. Bonding techniques used for joining of the transducer elements such as joining with glue, soldering, -

Redalyc.PHYSICAL-CHEMICAL PROPERTIES of BISMUTH AND
Dyna ISSN: 0012-7353 [email protected] Universidad Nacional de Colombia Colombia BEDOYA HINCAPIÉ, CLAUDIA MILENA; PINZÓN CÁRDENAS, MANUEL JONATHAN; ALFONSO ORJUELA, JOSE EDGAR; RESTREPO PARRA, ELISABETH; OLAYA FLOREZ, JHON JAIRO PHYSICAL-CHEMICAL PROPERTIES OF BISMUTH AND BISMUTH OXIDES: SYNTHESIS, CHARACTERIZATION AND APPLICATIONS Dyna, vol. 79, núm. 176, diciembre, 2012, pp. 139-148 Universidad Nacional de Colombia Medellín, Colombia Available in: http://www.redalyc.org/articulo.oa?id=49624953018 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative PHYSICAL-CHEMICAL PROPERTIES OF BISMUTH AND BISMUTH OXIDES: SYNTHESIS, CHARACTERIZATION AND APPLICATIONS PROPIEDADES FÍSICO-QUÍMICAS DEL BISMUTO Y OXIDOS DE BISMUTO: SÍNTESIS, CARACTERIZACIÓN Y APLICACIONES CLAUDIA MILENA BEDOYA HINCAPIÉ Ing. Física, Universidad Nacional de Colombia – Sede Bogotá, [email protected] MANUEL JONATHAN PINZÓN CÁRDENAS Ing. Mecatrónico, Universidad Nacional de Colombia – Sede Bogotá, [email protected] JOSE EDGAR ALFONSO ORJUELA PhD. Física, Universidad Nacional de Colombia – Sede Bogotá, [email protected] ELISABETH RESTREPO PARRA PhD. Ingeniería, Universidad Nacional de Colombia - Sede Manizales, [email protected] JHON JAIRO OLAYA FLOREZ PhD. Materiales, Universidad Nacional de Colombia - Sede Bogotá, [email protected] Received for review March 10 th, 2012, accepted June 29th, 2012, final version July, 9 th, 2012 ABSTRACT: The physical-chemical properties of bismuth and its oxides have been studied over the last two decades. As a result of this research, the growth of these materials with different crystallographic structures, showing micro and nanometric dimensions has been achieved by using several techniques (cathodic pulverization, laser pulsed deposition and hydrothermal method, among others). -

PVD Material Listing
P. O. Box 639 NL - 5550 AP Valkenswaard Tel: +31 (0)40 204 69 31 Fergutec Fax: +31 (0)40 201 39 81 E - mail: [email protected] PVD Material Listing Pure Metals Aluminum, Al Antimony, Sb Beryllium, Be Bismuth, Bi Boron, B Cadmium, Cd Calcium, Ca Carbon, C Cerium, Ce Chromium, Cr Cobalt, Co Copper, Cu Erbium, Er Gadolinium, Gd Gallium, Ga Germanium, Ge Gold, Au Hafnium, Hf Indium, In Iridium, Ir Iron, Fe Lanthanum, La Lead, Pb Magnesium, Mg Manganese, Mn Molybdenum, Mo Neodymium, Nd Nickel, Ni Niobium, Nb Osmium, Os Palladium, Pd Platinum, Pt Praseodymium, Pr Rhenium, Re Rhodium, Rh Ruthenium, Ru Samarium, Sm Selenium, Se Silicon, Si Silver, Ag Tantalum, Ta Fergutec b.v. P.O. Box 639, NL - 5550 AP Valkenswaard Heistraat 64, NL - 5554 ER Valkenswaard Bankaccount 45.80.36.714 ABN - AMRO Valkenswaard C.o.C. Eindhoven no. 17098554 VAT - ID NL8095.60.185.B01 The Standard Terms and Conditions, lodged at the Chamber of Commerce in Eindhoven, are applicable to all transactions. Tellurium, Te Terbium, Tb Tin, Sn Titanium, Ti Tungsten, W Vanadium, V Ytterbium, Yb Yttrium, Y Zinc, Zn Zirconium, Zr Precious Metals Gold Antimony, Au/Sb Gold Arsenic, Au/As Gold Boron, Au/B Gold Copper, Au/Cu Gold Germanium, Au/Ge Gold Nickel, Au/Ni Gold Nickel Indium, Au/Ni/In Gold Palladium, Au/Pd Gold Phosphorus, Au/P Gold Silicon, Au/Si Gold Silver Platinum, Au/Ag/Pt Gold Tantalum, Au/Ta Gold Tin, Au/Sn Gold Zinc, Au/Zn Palladium Lithium, Pd/Li Palladium Manganese, Pd/Mn Palladium Nickel, Pd/Ni Platinum Palladium, Pt/Pd Palladium Rhenium, Pd/Re Platinum Rhodium, -

High Purity Inorganics
High Purity Inorganics www.alfa.com INCLUDING: • Puratronic® High Purity Inorganics • Ultra Dry Anhydrous Materials • REacton® Rare Earth Products www.alfa.com Where Science Meets Service High Purity Inorganics from Alfa Aesar Known worldwide as a leading manufacturer of high purity inorganic compounds, Alfa Aesar produces thousands of distinct materials to exacting standards for research, development and production applications. Custom production and packaging services are part of our regular offering. Our brands are recognized for purity and quality and are backed up by technical and sales teams dedicated to providing the best service. This catalog contains only a selection of our wide range of high purity inorganic materials. Many more products from our full range of over 46,000 items are available in our main catalog or online at www.alfa.com. APPLICATION FOR INORGANICS High Purity Products for Crystal Growth Typically, materials are manufactured to 99.995+% purity levels (metals basis). All materials are manufactured to have suitably low chloride, nitrate, sulfate and water content. Products include: • Lutetium(III) oxide • Niobium(V) oxide • Potassium carbonate • Sodium fluoride • Thulium(III) oxide • Tungsten(VI) oxide About Us GLOBAL INVENTORY The majority of our high purity inorganic compounds and related products are available in research and development quantities from stock. We also supply most products from stock in semi-bulk or bulk quantities. Many are in regular production and are available in bulk for next day shipment. Our experience in manufacturing, sourcing and handling a wide range of products enables us to respond quickly and efficiently to your needs. CUSTOM SYNTHESIS We offer flexible custom manufacturing services with the assurance of quality and confidentiality. -

High Temperature Piezo V1.20.Pdf
HIGH TEMPERATURE ULTRASONIC GAS FLOW SENSOR BASED ON LEAD FREE PIEZOELECTRIC MATERIAL Dalibor Krsmanovic Darwin College University of Cambridge A dissertation submitted for the degree of Doctor of Philosophy at the University of Cambridge November 2009 Academic Supervisor: Dr R. V. Kumar Industrial Supervisor: W. Averdieck Declaration of Originality I am submitting this dissertation for the degree of Doctor of Philosophy at the University of Cambridge. The said thesis contains work I carried out between October 2006 and November 2009 at the Department of Materials Science and Metallurgy, University of Cambridge, under the academic supervision of Dr. R. V. Kumar. I state that, except where indicated, the work described is to the best of my knowledge, original and my own work. This dissertation does not exceed the word limit of 60,000 words. Dalibor Krsmanovic Darwin College, Cambridge i Abstract The review of current technologies for measurement of gas velocity in stack flow applications is undertaken and it is shown that the ultrasonic time-of-flight method is the most suitable and offers a number of advantages over alternatives. Weakness of current piezoelectric based transducers are identified as the inability to operate at temperatures above 400 °C due to limitation of piezoelectric materials used, and a case for development of an alternative high temperature material is put forward. A novel and highly enhanced, lead free piezoelectric material, suitable for continuous operation at temperatures above 400 °C has been engineered for ultrasonic gas velocity sensor applications. Structural modification of pure bismuth titanate (Bi4Ti3O12) or BIT compound, through multi-doping at the Ti-site, has been found to enhance piezoelectric properties accompanied with a mild reduction in Curie temperature, Tc. -

(12) United States Patent (10) Patent No.: US 8,709,304 B2 Subramanian Et Al
US0087.09304B2 (12) United States Patent (10) Patent No.: US 8,709,304 B2 Subramanian et al. (45) Date of Patent: Apr. 29, 2014 (54) HYDROTHERMAL SYNTHESIS OF Hinogami et al... An Approach to Ideal Smiconductor Electrodes for NANOCUBES OF SILLENITE TYPE Efficient Photoelectrochemical Reduction of Carbon Dixoide by Modification with Small Metal Particles, Journal of Physical Chem COMPOUNDS FORPHOTOVOLTAC istry B, 1998, pp. 974-980, vol. 102, No. 6. APPLICATIONS AND SOLAR ENERGY Matsumoto et al., Photocatalytic Reduction of Carbon Dioxide on CONVERSION OF CARBON DOXDE TO p-Type CaFe2O4 Powder, The Journal of Physical Chemistry, 1994, FUELS pp. 2950-2951, vol. 98, No. 11. Wang et al., Visible Light Photoreduction of CO2 Using CdSe/Pt? (75) Inventors: Vaidyanathan Subramanian, Sparks, TiO2 Heterostructured Catalysts, The Journal of Physical Chemistry NV (US); Sankaran Murugesan, Letters, 2010, pp. 48-53, vol. 1. Kocietal. Effect of TiO2 particle size on the photocatalytic reduc Austin, TX (US) tion of CO2, Applied Catalysis B. Environmental, 2009, pp. 494-502, vol. 89. (73) Assignee: Board of Regents of the Nevada Xie et al., Application of Surface photovoltage technique in System of Higher Education, on behalf photocatalysis studies on modified TiO2 photo-catalysts for photo of the University of Nevada, Reno, reduction of CO2, Materials Chemistry and Physics, 2001, pp. 103 Reno, NV (US) 106, vol. 70. Tseng et al., Effects of Sol-gel procedures on the photocatalysis of (*) Notice: Subject to any disclaimer, the term of this Cu/TiO2 in CO2 photoreduction, Journal of Catalysis, 2004, pp. patent is extended or adjusted under 35 432-440, vol. -

Angstrom Sciences PVD Materials
40 S. Linden Street, Duquesne, PA 15110 USA Tel: (412) 469-8466 Fax: (412) 469-8511 www.angstromsciences.com PVD Materials Target Sputtering Targets, Pellets, Bonding Services Slugs, Starter Charges, Crucibles Recycling of precious metal All Configurations of Clips, scrap Rod, Tube, pieces and Wire Pure Elements / Noble Metals Aluminum, (Al) Gadolinium, (Gd) Nickel, (Ni) Tantalum, (Ta) Antimony, (Sb) Gallium, (Ga) Niobium, (Nb) Tellurium, (Te) Beryllium, (Be) Germanium, (Ge) Osmium, (Os) Terbium, (Tb) Bismuth, (Bi) Gold, (Au) Palladium, (Pd) Tin, (Sn) Boron, (B) Hafnium, (Hf) Platinum, (Pt) Titanium, (Ti) Cadmium, (Cd) Indium, (In) Praseodymium, (Pr) Tungsten, (W) Calcium, (Ca) Iridium, (Ir) Rhenium, (Re) Vanadium, (V) Carbon, (C) Iron, (Fe) Rhodium, (Rh) Ytterbium, (Yb) Cerium, (Ce) Lanthanum, (La) Ruthenium, (Ru) Yttrium, (Y) Chromium, (Cr) Lead, (Pb) Samarium, (Sm) Zinc, (Zn) Cobalt, (Co) Magnesium, (Mg) Selenium, (Se) Zirconium, (Zr) Copper, (Cu) Manganese, (Mn) Silicon, (Si) Erbium, (Er) Molybdenum, (Mo) Silver, (Ag) Precious Metal Alloys Gold Antimony, (Au/Sb) Gold Zinc, (Au/Zn) Gold Boron, (Au/B) Palladium Manganese, (Pd/Mn) Gold Copper, (Au/Cu) Palladium Nickel, (Pd/Ni) Gold Germanium, (Au/Ge) Platinum Palladium, (Pt/Pd) Gold Nickel, (Au/Ni) Palladium Rhenium, (Pd/Re) Gold Nickel Indium, (Au/Ni/In) Platinum Rhodium, (Pt/Rh) Gold Palladium, (Au/Pd) Silver Copper, (Ag/Cu) Gold Silicon, (Au/Si) Silver Gallium, (Ag/Ga) Gold Silver Platinum, (Au/Ag/Pt) Silver Gold, (Ag/Au) Gold Tantalum, (Au/Ta) Silver Palladium, (Ag/Pd) Gold Tin, -

Our Coating Materials
Our coating materials MATERIALS COMPOSITION PLANAR ROTARY PURITIES TARGETS TARGETS METALS ALUMINUM Al x x 99.99- 99.9995 % ANTIMONY Sb x 99.99- 99.999 % BISMUTH Bi x 99.5, 99.999 % BORON B x 99.9 % CARBON C x 99.5, 99.999 % CERIUM Ce x 99.9, 99.99 % CHROMIUM Cr x x 99.8- 99.98 % COBALT Co x 99.8 % COPPER Cu x x 99.9, 99.9999 % ERBIUM Er x 99.9 % GERMANIUM Ge x 99.5, 99.999 % GOLD Au x x 99.99, 99.999 % HAFNIUM Hf x 99.9 % HOLMIUM Ho x 99.9 % INDIUM In x x 99.99- 99.9999 % IRIDIUM Ir x 99.95, 99.99 % IRON Fe x 99.9 % MAGNESIUM Mg x 99.9, 99.99 % MANGANESE Mn x 99.9 % MOLYBDENUM Mo x x 99.95 % NICKEL Ni x 99.7, 99.995 % NIOBIUM Nb x x 99.95 % www.fhr.biz Page 1 FHR Anlagenbau GmbH · Am Hügel 2 · 01458 Ottendorf-Okrilla (Germany) MATERIALS COMPOSITION PLANAR ROTARY PURITIES TARGETS TARGETS METALS PALLADIUM Pd x 99.95 % PLATINUM Pt x 99.99 % RHENIUM Re x 99.99 % RUTHENIUM Ru x 99.9 % SCANDIUM Sc x 99.9 % SILICON Si x x 99.99, 99.9999 % SILVER Ag x x 99.9, 99.99 % TANTALUM Ta x x 99.95 % TERBIUM Tb x 99.9 % TIN Sn x x 99.99, 99.999 % TITANIUM Ti x x 99.7- 99.995 % TUNGSTEN W x 99.95 % VANADIUM V x 99.8 % YTTRIUM Y x 99.9 % ZINC Zn x 99.99 % ZIRCONIUM Zr x 99.8 % ALLOYS ALUMINUM - CHROMIUM (Al)X (Cr)1-X x x 99.99 % ALUMINUM - COBALT (Al)X (Co)1-X x 99.95 % ALUMINUM- COPPER (Al)X (Cu)1-X x x 99.95 % ALUMINUM - IRON (Al)X (Fe)1-X x 99.99 % www.fhr.biz Page 2 MATERIALS COMPOSITION PLANAR ROTARY PURITIES TARGETS TARGETS ALLOYS ALUMINUM - SILICON (Al)X (Si)1-X x x 99.5- 99.95 % ALUMINUM - SILICON - COPPER (Al)X (Si)Y (Cu)Z x 99.5- 99.95 % ALUMINUM-MAGNESIUM -

Book of Abstracts
Eu IX International conferenceHe of young scientists on chemistry „Mendeleev- 2015” Eu Ca Be Al In He Ga Fr Os Np Md La Ti Se Ru Pd Mendeleev 2015 Book of abstracts 7-10 of April 2015 Saint Petersburg Saint Petersburg State University Mendeleev Russian Chemical Society Russian Foundation for Basic research “ELTECH” Creating high-tech fabrication facilities Institute of Chemistry IX INTERNATIONAL CONFERENCE OF YOUNG SCIENTISTS ON CHEMISTRY «MENDELEEV 2015» Book of abstracts Section 1 Inorganic materials and nanotechnology Section 2 Polymeric materials: methods of production and processing Section 3 Bioorganic and medical chemistry Section 4 Quantum chemistry and computer modeling Section 5 Modern techniques in analytical chemistry Saint Petersburg 2015 1 Mendeleev 2015. Section 1: Inorganic materials and nanotechnology; Section 2: Polymeric materials: methods of production and processing; Section 3: Bioorganic and medical chemistry; Section 4: Quantum chemistry and computer modeling; Section 5: Modern techniques in analytical chemistry. IX Iinternational conference of young scientists on chemistry. Book of abstracts. Saint Petersburg. 2015. ©Authors 2 CONFERENCE ORGANIZERS Saint Petersburg State University Mendeleev Russian Chemical Society Russian Foundation for Basic research “ELTECH” Creating high-tech fabrication facilities Institute of Chemistry Program committee: Chairman of the Program committee: Vadim Yu. Kukushkin, Professor, Dr.Sci., RAS Corresponding Member. Members of the Program committee: Evarestov R.A., Professor, Dr.Sci.; Islamova R.M., Professor, Dr.Sci.; Tverjanovich Yu.S., Professor, Dr.Sci.; Manshina A.A., Associate Professor, PhD; Vlakh E.G., Senior Researcher of Interdepartmental Laboratory of Biomedical Chemistry, Associate Professor, PhD; Vah K.S., Assistant professor, PhD; Novikov A.S., Postdoc, PhD. -

Barium and Zirconium Co-Doped Sodium Bismuth Titanate
Dielectric and Electromechanical Properties of Barium and Zirconium Co-Doped Sodium Bismuth Titanate by Sossity A. Sheets A.B. Earth Sciences, Dartmouth College, 1995 M.S. Earth Sciences, Dartmouth College, 1997 SUBMITTED TO THE DEPARTMENT OF MATERIALS SCIENCE AND ENGINEERING IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE IN MATERIALS SCIENCE AND ENGINEERING AT THE MASSACHUSETTS INSTITUTE OF TECHNOLOGY SEPTEMBER 2000 © 2000 Massachusetts Institute of Technology. All rights reserved. Signature of Author: 1 7 _· · · _ · Department of Materials Science and Engineering August 4, 2000 Certified by: _ J- /'et-Ming Chiang Kyocera Pr'fessor of Ceramics Thesis Supervisor C Accepted by: - I Carl V. Thompson Stavros Salapatas Professor of Materials Science & Engineering Chairman, Departmental Committee on Graduate Students MASSACHUSETTSINSTftE OF TECHNOLOGY I OCT 2 6 2004 ARCHIVES LIBRARIES Dielectric and Electromechanical Properties of Barium and Zirconium Co-Doped Sodium Bismuth Titanate by Sossity A. Sheets Submitted to the Department of Materials Science and Engineering on August 4, 2000 in Partial Fulfillment of the Requirements for the Degree of Master of Science in Materials Science and Engineering ABSTRACT Compositional exploration was conducted within the alkaline bismuth titanate system by doping on the A- and B- sites with Ba'2 and Zr'4, respectively. Results on the phase, dielectric and electromechanical properties of single crystals and polycrystals for this new family of relaxor perovskite ferroelectrics are presented. The actuation and polarization characteristics in this system were found to be highly sensitive (within 2 mol%) to cation doping levels, and tailored compositions successfully isolated predominantly electrostrictive actuation at room temperature. -
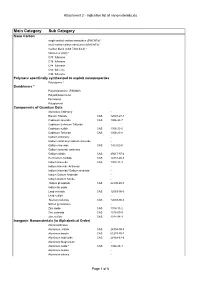
Attachment 2 - Indicative List of Nanomaterials.Xls
Attachment 2 - Indicative list of nanomaterials.xls Main Category Sub Category Nano Carbon single-walled carbon nanotubes (SWCNTs) * multi-walled carbon nanotubes (MWCNTs) * Carbon black (CAS 7440-44-0) * fullerenes (C60) * C70 fullerene C76 fullerene C84 fullerene C90 fullerene C94 fullerene Polymers specifically synthesized to exploit nanoproperties Polystyrene * Dendrimers * Polyamidoamine (PAMAM) Polyethylene imine Ferrocenyl Polyglycerol Components of Quantum Dots Aluminum Antinomy - Barium Titanate CAS 12047-27-7 Cadmium selenide CAS 1306-24-7 Cadmium Selenium Telluride Cadmium sulfide CAS 1306-23-6 Cadmium Telluride CAS 1306-25-8 Gallium Antimony - Gallium antimony/ Gallium arsenide - Gallium Arsenide CAS 1303-00-0 Gallium arsenide antimony Gallium nitride CAS 25617-97-4 Germanium carbide CAS 12334-26-8 Indium Arsenide CAS 1303-11-3 Indium Arsenide Antimony - Indium Arsenide/ Gallium arsenide - Indium Gallium Arsenide - Indium Gallium Nitride - Indium phosphide CAS 22398-80-7 Indium tin oxide - Lead selenide CAS 12069-00-0 Lead sulfide - Mercury telluride CAS 12068-90-5 Silicon germanium - Zinc oxide CAS 1314-13-2 Zinc selenide CAS 1315-09-9 Zinc sulfide CAS 1314-98-3 Inorganic Nanomaterials (in Alphabetical Order) Aluminosilicates - Aluminum nitride CAS 24304-00-5 Aluminum borate CAS 61279-70-7 Aluminum hydroxide CAS 20768-67-6 Aluminum Magnesium - Aluminum oxide * CAS 1344-28-1 Aluminum oxides - Aluminum silicate - Page 1 of 6 Attachment 2 - Indicative list of nanomaterials.xls Main Category Sub Category Aluminum Titanium Oxide