Structure and Physiology of Developing Neuromuscular Synapses in Culture
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cell Biology and Fundamentals in Histology - En-Cours-2018-Liepr1004 Liepr1004 Cell Biology and Fundamentals in 2018 Histology
Université catholique de Louvain - Cell biology and fundamentals in histology - en-cours-2018-liepr1004 liepr1004 Cell biology and fundamentals in 2018 histology 5 credits 45.0 h Q2 Teacher(s) Behets Wydemans Catherine ;Henriet Patrick ; Language : French Place of the course Louvain-la-Neuve Main themes The major themes are : - Characteristics common to all living species - The human cell, its functioning and division - Classical, evolutive and molecular genetics - Cellular bases in sexual reproduction - The differents cell types and their organisation in tissues - The major steps in human embryonic development Aims By the end of the module, students should understand the bases of unicity and diversity in the living world. They will know the structure and functioning of human cell and genome as well as the mechanisms of 1 cell division and embryonic development. Moreover, they will know the structure of the major types of human tissues. - - - - The contribution of this Teaching Unit to the development and command of the skills and learning outcomes of the programme(s) can be accessed at the end of this sheet, in the section entitled “Programmes/courses offering this Teaching Unit”. Content (auteurs - titulaires actuels) : P. Henriet and Ph. van den Bosch de Aguilar 1. UNICITY IN THE LIVING WORLD 2. THE HUMAN CELL 3. DIVERSITY IN THE LIVING WORLD 4. MOLECULAR GENETICS 5. CELL DIVISION 6. GAMETOGENESIS AND FERTILIZATION 7. INTRODUCTION TO HUMAN EMBRYOLOGY Histology 1. EPITHELIAL TISSUE 2. CONNECTIVE TISSUE 3. BLOOD TISSUE 4. MUSCLE TISSUE -

Immune Response and Histology of Humoral Rejection in Kidney
Document downloaded from http://www.elsevier.es, day 23/05/2017. This copy is for personal use. Any transmission of this document by any media or format is strictly prohibited. n e f r o l o g i a 2 0 1 6;3 6(4):354–367 Revista de la Sociedad Española de Nefrología www.revistanefrologia.com Review Immune response and histology of humoral rejection in kidney transplantation a,∗ a b a Miguel González-Molina , Pedro Ruiz-Esteban , Abelardo Caballero , Dolores Burgos , a c a a Mercedes Cabello , Miriam Leon , Laura Fuentes , Domingo Hernandez a Nephrology Department, Regional University Hospital of Malaga, Malaga University, IBIMA, REDINREN RD12/0021/0015, Malaga, Spain b Immunology Department, Regional University Hospital of Malaga, Malaga University, IBIMA, REDINREN RD12/0021/0015, Malaga, Spain c Pathology Department, Regional University Hospital of Malaga, Malaga University, IBIMA, REDINREN RD12/0021/0015, Malaga, Spain a r t i c l e i n f o a b s t r a c t Article history: The adaptive immune response forms the basis of allograft rejection. Its weapons are direct Received 4 June 2015 cellular cytotoxicity, identified from the beginning of organ transplantation, and/or anti- Accepted 26 March 2016 bodies, limited to hyperacute rejection by preformed antibodies and not as an allogenic Available online 3 June 2016 response. This resulted in allogenic response being thought for decades to have just a cellu- lar origin. But the experimental studies by Gorer demonstrating tissue damage in allografts Keywords: due to antibodies secreted by B lymphocytes activated against polymorphic molecules were Immune response disregarded. -

Satellitosis, a Crosstalk Between Neurons, Vascular Structures and Neoplastic Cells in Brain Tumours; Early Manifestation of Invasive Behaviour
cancers Review Satellitosis, a Crosstalk between Neurons, Vascular Structures and Neoplastic Cells in Brain Tumours; Early Manifestation of Invasive Behaviour Prospero Civita 1,2,* , Ortenzi Valerio 3 , Antonio Giuseppe Naccarato 3 , Mark Gumbleton 2 and Geoffrey J. Pilkington 1,2,4,* 1 Brain Tumour Research Centre, Institute of Biological and Biomedical Sciences (IBBS), School of Pharmacy and Biomedical Sciences, University of Portsmouth, Portsmouth PO1 2DT, UK 2 School of Pharmacy and Pharmaceutical Sciences, College of Biomedical and Life Sciences, Cardiff University, Cardiff CF10 3NB, UK; gumbleton@cardiff.ac.uk 3 Department of Translational Research and New Technologies in Medicine and Surgery, Pisa University Hospital, 56100 Pisa, Italy; [email protected] (O.V.); [email protected] (A.G.N.) 4 Division of Neuroscience, Department of Basic and Clinical Neuroscience, Institute of Psychiatry & Neurology, King’s College London, London SE5 9RX, UK * Correspondence: CivitaP@cardiff.ac.uk (P.C.); geoff[email protected] (G.J.P.) Received: 9 November 2020; Accepted: 4 December 2020; Published: 11 December 2020 Simple Summary: This article reviews the concept of cellular satellitosis as originally described histologically by Santiago Ramón y Cajal in 1899 and Hans Joachim Scherer, more specifically in the context of glioblastoma invasiveness, during the early part of the 20th century. With the advent of new and emerging molecular technologies in the 21st century, the significance of both vascular and neuronal satellitosis by neoplastic cells offers intriguing possibilities into further clarifying the development, pathobiology and therapy of malignant glioma through closer investigation into the nature of these histological hallmarks. -

Basic Histology (23 Questions): Oral Histology (16 Questions
Board Question Breakdown (Anatomic Sciences section) The Anatomic Sciences portion of part I of the Dental Board exams consists of 100 test items. They are broken up into the following distribution: Gross Anatomy (50 questions): Head - 28 questions broken down in this fashion: - Oral cavity - 6 questions - Extraoral structures - 12 questions - Osteology - 6 questions - TMJ and muscles of mastication - 4 questions Neck - 5 questions Upper Limb - 3 questions Thoracic cavity - 5 questions Abdominopelvic cavity - 2 questions Neuroanatomy (CNS, ANS +) - 7 questions Basic Histology (23 questions): Ultrastructure (cell organelles) - 4 questions Basic tissues - 4 questions Bone, cartilage & joints - 3 questions Lymphatic & circulatory systems - 3 questions Endocrine system - 2 questions Respiratory system - 1 question Gastrointestinal system - 3 questions Genitouirinary systems - (reproductive & urinary) 2 questions Integument - 1 question Oral Histology (16 questions): Tooth & supporting structures - 9 questions Soft oral tissues (including dentin) - 5 questions Temporomandibular joint - 2 questions Developmental Biology (11 questions): Osteogenesis (bone formation) - 2 questions Tooth development, eruption & movement - 4 questions General embryology - 2 questions 2 National Board Part 1: Review questions for histology/oral histology (Answers follow at the end) 1. Normally most of the circulating white blood cells are a. basophilic leukocytes b. monocytes c. lymphocytes d. eosinophilic leukocytes e. neutrophilic leukocytes 2. Blood platelets are products of a. osteoclasts b. basophils c. red blood cells d. plasma cells e. megakaryocytes 3. Bacteria are frequently ingested by a. neutrophilic leukocytes b. basophilic leukocytes c. mast cells d. small lymphocytes e. fibrocytes 4. It is believed that worn out red cells are normally destroyed in the spleen by a. neutrophils b. -

A System for Studying Mechanisms of Neuromuscular Junction Development and Maintenance Valérie Vilmont1,‡, Bruno Cadot1, Gilles Ouanounou2 and Edgar R
© 2016. Published by The Company of Biologists Ltd | Development (2016) 143, 2464-2477 doi:10.1242/dev.130278 TECHNIQUES AND RESOURCES RESEARCH ARTICLE A system for studying mechanisms of neuromuscular junction development and maintenance Valérie Vilmont1,‡, Bruno Cadot1, Gilles Ouanounou2 and Edgar R. Gomes1,3,*,‡ ABSTRACT different animal models and cell lines (Chen et al., 2014; Corti et al., The neuromuscular junction (NMJ), a cellular synapse between a 2012; Lenzi et al., 2015) with the hope of recapitulating some motor neuron and a skeletal muscle fiber, enables the translation of features of neuromuscular diseases and understanding the triggers chemical cues into physical activity. The development of this special of one of their common hallmarks: the disruption of the structure has been subject to numerous investigations, but its neuromuscular junction (NMJ). The NMJ is one of the most complexity renders in vivo studies particularly difficult to perform. studied synapses. It is formed of three key elements: the lower motor In vitro modeling of the neuromuscular junction represents a powerful neuron (the pre-synaptic compartment), the skeletal muscle (the tool to delineate fully the fine tuning of events that lead to subcellular post-synaptic compartment) and the Schwann cell (Sanes and specialization at the pre-synaptic and post-synaptic sites. Here, we Lichtman, 1999). The NMJ is formed in a step-wise manner describe a novel heterologous co-culture in vitro method using rat following a series of cues involving these three cellular components spinal cord explants with dorsal root ganglia and murine primary and its role is basically to ensure the skeletal muscle functionality. -

The Histology of the Neuromuscular Junction In
75 THE HISTOLOGY OF THE NEUROMUSCULAR JUNCTION Downloaded from https://academic.oup.com/brain/article/84/1/75/372729 by guest on 27 September 2021 IN DYSTROPHIA MYOTONICA BY VIOLET MACDERMOT Department of Neurology, St. Thomas' Hospital, London, S.E.I (1) INTRODUCTION DYSTROPJHC MYOTONICA is a familial disease affecting males and females, usually presenting in adult life, characterized by muscular wasting and weakness together with certain other features. The muscles mainly in- volved are the temporal, masseter, facial, sternomastoid and limb muscles, in the latter those mainly affected being peripheral in distribution. A widespread disorder of muscular contraction, myotonia, is also present but is noticed chiefly in the tongue and in the muscles involved in grasping. The other features of the condition are some degree of mental defect, dysphonia, cataracts, frontal baldness, sparse body hair and testicular atrophy. Any of the manifestations of the disease may be absent and the order of presentation of symptoms is variable. The myotonia may precede muscular wasting by many years or may occur independently. In those muscles which are severely wasted the myotonia tends to disappear. The interest of dystrophia myotonica lies in the peculiar distribution of muscle involvement and in the combination of a disorder of muscle function with endocrine and other dysplasic features. The results of histological examination of biopsy and post-mortem material have been described and reviewed by numerous workers, notably Steinert (1909), Adie and Greenfield (1923), Keschner and Davison (1933), Hassin and Kesert (1948), Wohlfart (1951), Adams, Denny-Brown and Pearson (1953), Greenfield, Shy, Alvord and Berg (1957). -

Quiescent Satellite Glial Cells of the Adult Trigeminal Ganglion
Cent. Eur. J. Med. • 9(3) • 2014 • 500-504 DOI: 10.2478/s11536-013-0285-z Central European Journal of Medicine Quiescent satellite glial cells of the adult trigeminal ganglion Research Article Mugurel C. Rusu*1,2,3, Valentina M. Mănoiu4, Nicolae Mirancea3, Gheorghe Nini5 1 „Carol Davila” University of Medicine and Pharmacy, 050511 Bucharest, Romania. 2 MEDCENTER - Center of Excellence in Laboratory Medicine and Pathology 013594 Bucharest, Romania 3 Institute of Biology of Bucharest – The Romanian Academy, , 060031 Bucharest, Romania 4 Faculty of Geography, University of Bucharest, 050107 Bucharest, Romania 5 Faculty of Medicine, Pharmacy and Dental Medicine, “Vasile Goldiş” Western University, 310045 Arad, Romania Received 18 August 2013; Accepted 27 November 2013 Abstract: Sensory ganglia comprise functional units built up by neurons and satellite glial cells (SGCs). In animal species there was proven the presence of neuronoglial progenitor cells in adult samples. Such neural crest-derived progenitors were found in immunohistochemistry (IHC). These fi ndings were not previously documented in transmission electron microscopy (TEM). It was thus aimed to assess in TEM if cells of the human adult trigeminal ganglion indeed have ultrastructural features to qualify for a progenitor, or quiescent phenotype. Trigeminal ganglia were obtained from fi fteen adult donor cadavers. In TEM, cells with heterochromatic nuclei, a pancytoplasmic content of free ribosomes, few perinuclear mitochondria, poor developed endoplasmic reticulum, lack of Golgi complexes and membrane traffi cking specializations, were found included in the neuronal envelopes built-up by SGCs. The ultrastructural pattern was strongly suggestive for these cells being quiescent progenitors. However, further experiments should correlate the morphologic and immune phenotypes of such cells. -
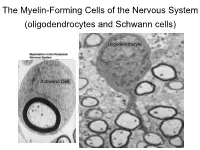
The Myelin-Forming Cells of the Nervous System (Oligodendrocytes and Schwann Cells)
The Myelin-Forming Cells of the Nervous System (oligodendrocytes and Schwann cells) Oligodendrocyte Schwann Cell Oligodendrocyte function Saltatory (jumping) nerve conduction Oligodendroglia PMD PMD Saltatory (jumping) nerve conduction Investigating the Myelinogenic Potential of Individual Oligodendrocytes In Vivo Sparse Labeling of Oligodendrocytes CNPase-GFP Variegated expression under the MBP-enhancer Cerebral Cortex Corpus Callosum Cerebral Cortex Corpus Callosum Cerebral Cortex Caudate Putamen Corpus Callosum Cerebral Cortex Caudate Putamen Corpus Callosum Corpus Callosum Cerebral Cortex Caudate Putamen Corpus Callosum Ant Commissure Corpus Callosum Cerebral Cortex Caudate Putamen Piriform Cortex Corpus Callosum Ant Commissure Characterization of Oligodendrocyte Morphology Cerebral Cortex Corpus Callosum Caudate Putamen Cerebellum Brain Stem Spinal Cord Oligodendrocytes in disease: Cerebral Palsy ! CP major cause of chronic neurological morbidity and mortality in children ! CP incidence now about 3/1000 live births compared to 1/1000 in 1980 when we started intervening for ELBW ! Of all ELBW {gestation 6 mo, Wt. 0.5kg} , 10-15% develop CP ! Prematurely born children prone to white matter injury {WMI}, principle reason for the increase in incidence of CP ! ! 12 Cerebral Palsy Spectrum of white matter injury ! ! Macro Cystic Micro Cystic Gliotic Khwaja and Volpe 2009 13 Rationale for Repair/Remyelination in Multiple Sclerosis Oligodendrocyte specification oligodendrocytes specified from the pMN after MNs - a ventral source of oligodendrocytes -

Canine Dorsal Root Ganglia Satellite Glial Cells Represent an Exceptional Cell Population with Astrocytic and Oligodendrocytic P
www.nature.com/scientificreports OPEN Canine dorsal root ganglia satellite glial cells represent an exceptional cell population with astrocytic and Received: 17 August 2017 Accepted: 6 October 2017 oligodendrocytic properties Published: xx xx xxxx W. Tongtako1,2, A. Lehmbecker1, Y. Wang1,2, K. Hahn1,2, W. Baumgärtner1,2 & I. Gerhauser 1 Dogs can be used as a translational animal model to close the gap between basic discoveries in rodents and clinical trials in humans. The present study compared the species-specifc properties of satellite glial cells (SGCs) of canine and murine dorsal root ganglia (DRG) in situ and in vitro using light microscopy, electron microscopy, and immunostainings. The in situ expression of CNPase, GFAP, and glutamine synthetase (GS) has also been investigated in simian SGCs. In situ, most canine SGCs (>80%) expressed the neural progenitor cell markers nestin and Sox2. CNPase and GFAP were found in most canine and simian but not murine SGCs. GS was detected in 94% of simian and 71% of murine SGCs, whereas only 44% of canine SGCs expressed GS. In vitro, most canine (>84%) and murine (>96%) SGCs expressed CNPase, whereas GFAP expression was diferentially afected by culture conditions and varied between 10% and 40%. However, GFAP expression was induced by bone morphogenetic protein 4 in SGCs of both species. Interestingly, canine SGCs also stimulated neurite formation of DRG neurons. These fndings indicate that SGCs represent an exceptional, intermediate glial cell population with phenotypical characteristics of oligodendrocytes and astrocytes and might possess intrinsic regenerative capabilities in vivo. Since the discovery of glial cells over a century ago, substantial progress has been made in understanding the origin, development, and function of the diferent types of glial cells in the central nervous system (CNS) and peripheral nervous system (PNS)1. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

Specific Labeling of Synaptic Schwann Cells Reveals Unique Cellular And
RESEARCH ARTICLE Specific labeling of synaptic schwann cells reveals unique cellular and molecular features Ryan Castro1,2,3, Thomas Taetzsch1,2, Sydney K Vaughan1,2, Kerilyn Godbe4, John Chappell4, Robert E Settlage5, Gregorio Valdez1,2,6* 1Department of Molecular Biology, Cellular Biology, and Biochemistry, Brown University, Providence, United States; 2Center for Translational Neuroscience, Robert J. and Nancy D. Carney Institute for Brain Science and Brown Institute for Translational Science, Brown University, Providence, United States; 3Neuroscience Graduate Program, Brown University, Providence, United States; 4Fralin Biomedical Research Institute at Virginia Tech Carilion, Roanoke, United States; 5Department of Advanced Research Computing, Virginia Tech, Blacksburg, United States; 6Department of Neurology, Warren Alpert Medical School of Brown University, Providence, United States Abstract Perisynaptic Schwann cells (PSCs) are specialized, non-myelinating, synaptic glia of the neuromuscular junction (NMJ), that participate in synapse development, function, maintenance, and repair. The study of PSCs has relied on an anatomy-based approach, as the identities of cell-specific PSC molecular markers have remained elusive. This limited approach has precluded our ability to isolate and genetically manipulate PSCs in a cell specific manner. We have identified neuron-glia antigen 2 (NG2) as a unique molecular marker of S100b+ PSCs in skeletal muscle. NG2 is expressed in Schwann cells already associated with the NMJ, indicating that it is a marker of differentiated PSCs. Using a newly generated transgenic mouse in which PSCs are specifically labeled, we show that PSCs have a unique molecular signature that includes genes known to play critical roles in *For correspondence: PSCs and synapses. These findings will serve as a springboard for revealing drivers of PSC [email protected] differentiation and function. -

Pretest Anatomy, Histology & Cell Biology
Anatomy, Histology, and Cell Biology PreTestTMSelf-Assessment and Review Notice Medicine is an ever-changing science. As new research and clinical experience broaden our knowledge, changes in treatment and drug therapy are required. The editors and the publisher of this work have checked with sources believed to be reli- able in their efforts to provide information that is complete and generally in accord with the standards accepted at the time of publication. However, in view of the pos- sibility of human error or changes in medical sciences, neither the editors nor the publisher nor any other party who has been involved in the preparation or publi- cation of this work warrants that the information contained herein is in every respect accurate or complete, and they are not responsible for any errors or omis- sions or for the results obtained from use of such information. Readers are encour- aged to confirm the information contained herein with other sources. For example and in particular, readers are advised to check the product information sheet included in the package of each drug they plan to administer to be certain that the information contained in this book is accurate and that changes have not been made in the recommended dose or in the contraindications for administration. This recommendation is of particular importance in connection with new or infre- quently used drugs. Anatomy, Histology, and Cell Biology PreTestTMSelf-Assessment and Review Third Edition Robert M. Klein, PhD Professor and Associate Dean Professional Development and Faculty Affairs Department of Anatomy and Cell Biology University of Kansas, School of Medicine Kansas City, Kansas George C.