Eastern Brown Snake (Pseudonaja Textilis) Envenomation in Dogs And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Guidelines for Keeping Venomous Snakes in the NT
GUIDELINES FOR KEEPING VENOMOUS SNAKES IN THE NT Venomous snakes are potentially dangerous to humans, and for this reason extreme caution must be exercised when keeping or handling them in captivity. Prospective venomous snake owners should be well informed about the needs and requirements for keeping these animals in captivity. Permits The keeping of protected wildlife in the Northern Territory is regulated by a permit system under the Territory Parks and Wildlife Conservation Act 2006 (TPWC Act). Conditions are included on permits, and the Parks and Wildlife Commission of the Northern Territory (“PWCNT”) may issue infringement notices or cancel permits if conditions are breached. A Permit to Keep Protected Wildlife enables people to legally possess native vertebrate animals in captivity in the Northern Territory. The permit system assists the PWCNT to monitor wildlife kept in captivity and to detect any illegal activities associated with the keeping of, and trade in, native wildlife. Venomous snakes are protected throughout the Northern Territory and may not be removed from the wild without the appropriate licences and permits. People are required to hold a Keep Permit (Category 1–3) to legally keep venomous snakes in the Northern Territory. Premises will be inspected by PWCNT staff to evaluate their suitability prior to any Keep Permit (Category 1– 3) being granted. Approvals may also be required from local councils, the Northern Territory Planning Authority, and the Department of Health and Community Services. Consignment of venomous snakes between the Northern Territory and other States and Territories can only be undertaken with an appropriate import / export permit. There are three categories of venomous snake permitted to be kept in captivity in the Northern Territory: Keep Permit (Category 1) – Mildly Dangerous Venomous Keep Permit (Category 2) – Dangerous Venomous Keep Permit (Category 3) – Highly Dangerous Venomous Venomous snakes must be obtained from a legal source (i.e. -

Draft Animal Keepers Species List
Revised NSW Native Animal Keepers’ Species List Draft © 2017 State of NSW and Office of Environment and Heritage With the exception of photographs, the State of NSW and Office of Environment and Heritage are pleased to allow this material to be reproduced in whole or in part for educational and non-commercial use, provided the meaning is unchanged and its source, publisher and authorship are acknowledged. Specific permission is required for the reproduction of photographs. The Office of Environment and Heritage (OEH) has compiled this report in good faith, exercising all due care and attention. No representation is made about the accuracy, completeness or suitability of the information in this publication for any particular purpose. OEH shall not be liable for any damage which may occur to any person or organisation taking action or not on the basis of this publication. Readers should seek appropriate advice when applying the information to their specific needs. All content in this publication is owned by OEH and is protected by Crown Copyright, unless credited otherwise. It is licensed under the Creative Commons Attribution 4.0 International (CC BY 4.0), subject to the exemptions contained in the licence. The legal code for the licence is available at Creative Commons. OEH asserts the right to be attributed as author of the original material in the following manner: © State of New South Wales and Office of Environment and Heritage 2017. Published by: Office of Environment and Heritage 59 Goulburn Street, Sydney NSW 2000 PO Box A290, -

Reptiles in and Around the House Identification and Distribution Reptiles Inhabit Every Environment in Australia
Reptiles in and around the house Identification and Distribution Reptiles inhabit every environment in Australia. Common reptiles found in Western Australian backyards include: Tiger snakes Notechis scutatus occur in southwest WA, and are often seen near water, including rivers, dams, drains and wetlands. Unlike most other Australian elapids, tiger snakes climb well. They can range from grey, olive, brown to black in colour and often have yellow and black cross-bands, but not all have this pattern. Venomous Dugite. Photo: R. Lloyd/Fauna Track Dugites Pseudonaja affinis occur in southwest WA and Gwarda Pseudonaja nuchalis occur from Perth northwards. They live in a wide variety of habitats including coastal dunes, heathlands, shrublands, woodlands and forests. They are long and slender, with relatively large scales that have a semi-glossy appearance. They can range from brown, olive to grey in colour, and can have irregular black/dark grey spotting, but patterning varies. Venomous Mulga snakes Pseudechis australis occur in a wide variety of habitats, northwards from Perth and Narrogin. They are quite robust, with a broad, deep head and bulbous cheeks. They can range from pale brown, dark olive to reddish-brown in colour, and darker snakes often have two-toned scales with a lighter colour that contrasts with the darker colour to produce a reticulated effect. The belly is cream to salmon-coloured. Venomous There are two subspecies of carpet pythons found in a large variety of habitats in WA: Morelia spilota imbricata occurs in the southwest and Morelia spilota variegata occurs in the Kimberley. They are 1-4m in length, tend to be pale to dark brown with black blotches that sometimes have a Carpet python. -

Venemous Snakes
WASAH WESTERN AUSTRALIAN SOCIETY of AMATEUR HERPETOLOGISTS (Inc) K E E P I N G A D V I C E S H E E T Venomous Snakes Southern Death Adder (Acanthophis Southern Death antarcticus) – Maximum length 100 cm. Adder Category 5. Desert Death Adder (Acanthophis pyrrhus) – Acanthophis antarcticus Maximum length 75 cm. Category 5. Pilbara Death Adder (Acanthophis wellsi) – Maximum length 70 cm. Category 5. Western Tiger Snake (Notechis scutatus) - Maximum length 160 cm. Category 5. Mulga Snake (Pseudechis australis) – Maximum length 300 cm. Category 5. Spotted Mulga Snake (Pseudechis butleri) – Maximum length 180 cm. Category 5. Dugite (Pseudonaja affinis affinis) – Maximum Desert Death Adder length 180 cm. Category 5. Acanthophis pyrrhus Gwardar (Pseudonaja nuchalis) – Maximum length 100 cm. Category 5. NOTE: All species listed here are dangerously venomous and are listed as Category 5. Only the experienced herpetoculturalist should consider keeping any of them. One must be over 18 years of age to hold a category 5 license. Maintaining a large elapid carries with 1 it a considerable responsibility. Unless you are Pilbara Death Adder confident that you can comply with all your obligations and licence requirements when Acanthophis wellsi keeping dangerous animals, then look to obtaining a non-venomous species instead. NATURAL HABITS: Venomous snakes occur in a wide variety of habitats and, apart from death adders, are highly mobile. All species are active day and night. HOUSING: In all species listed except death adders, one adult (to 150 cm total length) can be kept indoors in a lockable, top-ventilated, all glass or glass-fronted wooden vivarium of Western Tiger Snake at least 90 x 45 cm floor area. -

Does Urbanization Influence the Diet of a Large Snake?
Current Zoology, 2018, 64(3), 311–318 doi: 10.1093/cz/zox039 Advance Access Publication Date: 27 June 2017 Article Article Does urbanization influence the diet of a large snake? a, a b Ashleigh K. WOLFE *, Philip W. BATEMAN , and Patricia A. FLEMING aDepartment of Environment and Agriculture, Curtin University, Perth, Bentley, WA, 6102, Australia and bSchool of Veterinary and Life Sciences, Murdoch University, Perth, Murdoch, WA, 6150, Australia *Address correspondence to Ashleigh K. Wolfe. E-mail: [email protected] Received on 10 April 2017; accepted on 21 May 2017 Abstract Urbanization facilitates synanthropic species such as rodents, which benefit the diets of many preda- tors in cities. We investigated how urbanization affects the feeding ecology of dugites Pseudonaja affi- nis, a common elapid snake in south-west Western Australia. We predicted that urban snakes: 1) more frequently contain prey and eat larger meals, 2) eat proportionally more non-native prey, 3) eat a lower diversity of prey species, and 4) are relatively heavier, than non-urban dugites. We analyzed the diet of 453 specimens obtained from the Western Australian Museum and opportunistic road-kill collections. Correcting for size, sex, season, and temporal biases, we tested whether location influenced diet for our 4 predictions. Body size was a strong predictor of diet (larger snakes had larger prey present, a greater number of prey items, and a greater diversity of prey). We identified potential collection biases: urban dugites were relatively smaller (snout-vent length) than non-urban specimens, and females were relatively lighter than males. Accounting for these effects, urban snakes were less likely to have prey present in their stomachs and were relatively lighter than non-urban snakes. -

Berriquin LWMP Wildlife
Berriquin Wildlife Murray Land & Water Management Plan Wildlife Survey 2005-2006 Matthew Herring David Webb Michael Pisasale INTRODUCTION Why do a wildlife survey? 106 farms and were surveyed One of the great things about between June 2005 and March living in rural Australia is all the 2006. They incorporated a range wildlife that we share the land- of vegetation types (e.g. Black scape with. Historically, humans Box Woodland) as well as reveg- have impacted on the survival of etation on previously cleared many native plants and animals. land and constructed wetlands. Fortunately, there is a grow- Methods used to survey wildlife ing commitment in the country included: to wildlife conservation on the farm. As we improve our knowl- - Bird surveys edge and understanding of the - Log rolling for reptiles and local landscape and the animals frogs and plants that live in it we will - Spotlighting for mammals, rep be in a much better position to tiles and nocturnal birds conserve and enhance our natu- - Elliot traps for small mammals ral heritage for future genera- and reptiles tions. - Pitfall trapping for reptiles and frogs This wildlife survey was an ini- - Harp traps for bats tiative of the Berriquin Land & - Using the “Anabat” to record Water Management Plan (LWMP) bat calls M.Herring Working Group and is the largest - Call broadcasting to attract Wildlife expert Adam Bester and most extensive ever un- birds with 11 Little Forest Bats, one dertaken in the area. Berriquin of Berriquin’s most abundant was one of four LWMP areas that Other targeted methods were mammals. -

The Brown Snake Complex Genus Pseudonaja Formally Demansia
The Brown Snake Complex Genus Pseudonaja formally Demansia All Brown Snakes are egg layers not live bearers and all but one species the Ringed Brown (Pseudonaja modesta) should be classed as deadly. Specific antivenom is Brown snake antivenom. The Common or Eastern Brown (Pseudonaja textilis textilis) Found over much of Victoria especially in the drier areas. It is to be found in the south eastern corner of South Australia and most of New South Wales. Its range covers most of Queensland and has been recorded in a few spots of the Northern Territory; it is also found in parts of New Guinea. The venom of this snake is strongly coagulant and neurotoxic though other toxins are also present. The Eastern brown usually produces from 10 to 30 eggs. Egg counts will usually depend on the size of the snake. Browns are quite common in the wheat and other grain growing areas and their numbers have multiplied to take advantage of the mice that follow the grain. Browns being rather slender snakes can get their heads down mouse burrows or into places where mice would otherwise be safe. The Browns eat all the mice meaning the whole family. After taking care of the larger mice they’ll consume all the baby mice. If the mother gets away and these baby mice were left then she would return and rear them. Within a few more weeks all the females would be pregnant and then they would of course, start to breed like mice. My belief is that without Brown snakes in Australia we would find it very difficult to grow any grain (cereal) crops at all. -

Myths Surrounding Snakes
MYTHS SURROUNDING SNAKES MYTH 1: Bites from baby venomous snakes are more dangerous than those from adults because they always deliver a full dose of venom. The legend goes that young snakes have not yet learned how to control the amount of venom they inject. They are therefore more dangerous than adult snakes, which will restrict the amount of venom they use in a bite or “dry bite”. This is simply untrue and all the evidence points towards bites from adults being more severe. Tests have shown that juvenile snakes can control their venom just as much as adults. Furthermore lets consider the following factors: adults have significantly larger fangs to deliver their venom and considerably more venom available than a juvenile. Therefore if a juvenile has venom glands only big enough to hold a 2ml of venom compared to an adult that can hold 30ml or more, then the bite from an adult will always have the potential to be more severe. I presume the reason this myth came into existence was to dissuade people from having a carefree attitude towards the potential dangers of a juvenile snake. The moral of the story is to treat every snake as a potentially dangerous and never expose your self to a situation where a snake of any size can bite you. MYTH 2: If you see a snake they’ll always be more Although it is possible to see more than one snake, for the most part this statement is untrue. Snakes are solitary animals for most of their lives so generally you will only ever encounter individuals. -

Taxonomy of the Genus Pseudonaja (Reptilia: Elapidae) in Australia
AUSTRALIAN BIODIVERSITY RECORD ________________________________________________________ 2002 (No 7) ISSN 1325-2992 March, 2002 ________________________________________________________ Taxonomy of the Genus Pseudonaja (Reptilia: Elapidae) in Australia. by Richard W. Wells “Shiralee”, Major West Road, Cowra, New South Wales, Australia The clear morphological differences that exist within the genus as previously considered strongly indicate that it is a polyphyletic assemblage. Accordingly, I have taken the step of formally proposing the fragmentation of Pseudonaja. In this work I have decided to restrict the genus Pseudonaja to the Pseudonaja nuchalis complex. Additionally, I herein formally resurrect from synonymy the generic name Euprepiosoma Fitzinger, 1860 for the textilis group of species, erect a new generic name (Placidaserpens gen. nov.) for the snakes previously regarded as Pseudonaja guttata, erect a new generic name (Notopseudonaja gen. nov.) for the group of species previously regarded as the Pseudonaja modesta complex, and erect a new generic name (Dugitophis gen. nov.) for snakes previously regarded as the Pseudonaja affinis complex. Genus Pseudonaja Gunther, 1858 The Pseudonaja nuchalis Complex It is usually reported that Pseudonaja nuchalis occurs across most of northern, central and western Australia, ranging from Cape York Peninsula, in the north-east, through western, southern and south-eastern Queensland, far western New South Wales, north-western Victoria, and most of South Australia, Northern Territory and Western Australia. However, this distribution pattern is now known to actually represents several different species all regarded by most authorities for convenience as the single highly variable species, 'Pseudonaja nuchalis'. As usually defined, this actually is a highly variable and therefore confusing group of species to identify and it is not all surprising that there has been difficulty in breaking up the group. -

Very Venomous, But...- Snakes of the Wet Tropics
No.80 January 2004 Notes from Very venomous but ... the Australia is home to some of the most venomous snakes in the world. Why? Editor It is possible that strong venom may little chance to fight back. There are six main snake families have evolved chiefly as a self-defence in Australia – elapids (venomous strategy. It is interesting to look at the While coastal and inland taipans eat snakes, the largest group), habits of different venomous snakes. only mammals, other venomous colubrids (‘harmless’ snakes) Some, such as the coastal taipan snakes feed largely on reptiles and pythons, blindsnakes, filesnakes (Oxyuranus scutellatus), bite their frogs. Venom acts slowly on these and seasnakes. prey quickly, delivering a large amount ‘cold-blooded’ creatures with slow of venom, and then let go. The strong metabolic rates, so perhaps it needs to Australia is the only continent venom means that the prey doesn’t be especially strong. In addition, as where venomous snakes (70 get far before succumbing so the many prey species develop a degree of percent) outnumber non- snake is able to follow at a safe immunity to snake venom, a form of venomous ones. Despite this, as distance. Taipans eat only mammals – evolutionary arms race may have been the graph on page one illustrates, which are able to bite back, viciously. taking place. very few deaths result from snake This strategy therefore allows the bites. It is estimated that between snake to avoid injury. … not necessarily deadly 50 000 and 60 000 people die of On the other hand, the most Some Australian snakes may be snake bite each year around the particularly venomous, but they are world. -
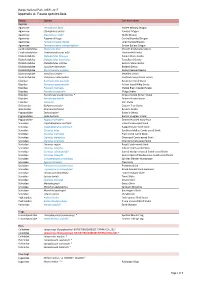
Report-Mungo National Park-Appendix A
Mungo National Park, NSW, 2017 Appendix A: Fauna species lists Family Species Common name Reptiles Agamidae Ctenophorus fordi Mallee Military Dragon Agamidae Ctenophorus pictus Painted Dragon Agamidae Diporiphora nobbi Nobbi Dragon Agamidae Pogona vitticeps Central Bearded Dragon Agamidae Tympanocryptis lineata Lined Earless Dragon Agamidae Tympanocryptis tetraporophora Eyrean Earless Dragon Carphodactylidae Nephrurus levis Smooth Knob-tailed Gecko Carphodactylidae Underwoodisaurus milii Thick-tailed Gecko Diplodactylidae Diplodactylus furcosus Ranges Stone Gecko Diplodactylidae Diplodactylus tessellatus Tessellated Gecko Diplodactylidae Diplodactylus vittatus Eastern Stone Gecko Diplodactylidae Lucasium damaeum Beaded Gecko Diplodactylidae Rhynchoedura ormsbyi Eastern Beaked Gecko Diplodactylidae Strophurus elderi ~ Jewelled Gecko Diplodactylidae Strophurus intermedius Southern Spiny-tailed Gecko Elapidae Brachyurophis australis Australian Coral Snake Elapidae Demansia psammophis Yellow-faced Whip Snake Elapidae Parasuta nigriceps Mallee Black-headed Snake Elapidae Pseudechis australis Mulga Snake Elapidae Pseudonaja aspidorhyncha * Strap-snouted Brown Snake Elapidae Pseudonaja textilis Eastern Brown Snake Elapidae Suta suta Curl Snake Gekkonidae Gehyra versicolor Eastern Tree Gecko Gekkonidae Heteronotia binoei Bynoe's Gecko Pygopodidae Delma butleri Butler's Delma Pygopodidae Lialis burtonis Burton's Legless Lizard Pygopodidae Pygopus schraderi Eastern Hooded Scaly-foot Scincidae Cryptoblepharus australis Inland Snake-eyed Skink Scincidae -

Comparative Studies of the Venom of a New Taipan Species, Oxyuranus Temporalis, with Other Members of Its Genus
Toxins 2014, 6, 1979-1995; doi:10.3390/toxins6071979 OPEN ACCESS toxins ISSN 2072-6651 www.mdpi.com/journal/toxins Article Comparative Studies of the Venom of a New Taipan Species, Oxyuranus temporalis, with Other Members of Its Genus Carmel M. Barber 1, Frank Madaras 2, Richard K. Turnbull 3, Terry Morley 4, Nathan Dunstan 5, Luke Allen 5, Tim Kuchel 3, Peter Mirtschin 2 and Wayne C. Hodgson 1,* 1 Monash Venom Group, Department of Pharmacology, Faculty of Medicine, Nursing and Health Sciences, Monash University, Clayton, Victoria 3168, Australia; E-Mail: [email protected] 2 Venom Science Pty Ltd, Tanunda, South Australia 5352, Australia; E-Mails: [email protected] (F.M.); [email protected] (P.M.) 3 SA Pathology, IMVS Veterinary Services, Gilles Plains, South Australia 5086, Australia; E-Mails: [email protected] (R.K.T.); [email protected] (T.K.) 4 Adelaide Zoo, Adelaide, South Australia 5000, Australia; E-Mail: [email protected] 5 Venom Supplies, Tanunda, South Australia, South Australia 5352, Australia; E-Mails: [email protected] (N.D.); [email protected] (L.A.) * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +61-3-9905-4861; Fax: +61-3-9905-2547. Received: 5 May 2014; in revised form: 11 June 2014 / Accepted: 16 June 2014 / Published: 2 July 2014 Abstract: Taipans are highly venomous Australo-Papuan elapids. A new species of taipan, the Western Desert Taipan (Oxyuranus temporalis), has been discovered with two specimens housed in captivity at the Adelaide Zoo. This study is the first investigation of O.