Chapter 7:Field Insect Pests Belowground and Seedling Feeders
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Distributional Study of the Butterflies of the Sierra De Tuxtla in Veracruz, Mexico. Gary Noel Ross Louisiana State University and Agricultural & Mechanical College
Louisiana State University LSU Digital Commons LSU Historical Dissertations and Theses Graduate School 1967 A Distributional Study of the Butterflies of the Sierra De Tuxtla in Veracruz, Mexico. Gary Noel Ross Louisiana State University and Agricultural & Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_disstheses Recommended Citation Ross, Gary Noel, "A Distributional Study of the Butterflies of the Sierra De Tuxtla in Veracruz, Mexico." (1967). LSU Historical Dissertations and Theses. 1315. https://digitalcommons.lsu.edu/gradschool_disstheses/1315 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Historical Dissertations and Theses by an authorized administrator of LSU Digital Commons. For more information, please contact [email protected]. This dissertation has been microfilmed exactly as received 67-14,010 ROSS, Gary Noel, 1940- A DISTRIBUTIONAL STUDY OF THE BUTTERFLIES OF THE SIERRA DE TUXTLA IN VERACRUZ, MEXICO. Louisiana State University and Agricultural and Mechanical CoUege, Ph.D., 1967 Entomology University Microfilms, Inc., Ann Arbor, Michigan A DISTRIBUTIONAL STUDY OF THE BUTTERFLIES OF THE SIERRA DE TUXTLA IN VERACRUZ, MEXICO A D issertation Submitted to the Graduate Faculty of the Louisiana State University and A gricultural and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Entomology by Gary Noel Ross M.S., Louisiana State University, 196*+ May, 1967 FRONTISPIECE Section of the south wall of the crater of Volcan Santa Marta. May 1965, 5,100 feet. ACKNOWLEDGMENTS Many persons have contributed to and assisted me in the prep aration of this dissertation and I wish to express my sincerest ap preciation to them all. -

Origins of Six Species of Butterflies Migrating Through Northeastern
diversity Article Origins of Six Species of Butterflies Migrating through Northeastern Mexico: New Insights from Stable Isotope (δ2H) Analyses and a Call for Documenting Butterfly Migrations Keith A. Hobson 1,2,*, Jackson W. Kusack 2 and Blanca X. Mora-Alvarez 2 1 Environment and Climate Change Canada, 11 Innovation Blvd., Saskatoon, SK S7N 0H3, Canada 2 Department of Biology, University of Western Ontario, Ontario, ON N6A 5B7, Canada; [email protected] (J.W.K.); [email protected] (B.X.M.-A.) * Correspondence: [email protected] Abstract: Determining migratory connectivity within and among diverse taxa is crucial to their conservation. Insect migrations involve millions of individuals and are often spectacular. However, in general, virtually nothing is known about their structure. With anthropogenically induced global change, we risk losing most of these migrations before they are even described. We used stable hydrogen isotope (δ2H) measurements of wings of seven species of butterflies (Libytheana carinenta, Danaus gilippus, Phoebis sennae, Asterocampa leilia, Euptoieta claudia, Euptoieta hegesia, and Zerene cesonia) salvaged as roadkill when migrating in fall through a narrow bottleneck in northeast Mexico. These data were used to depict the probabilistic origins in North America of six species, excluding the largely local E. hegesia. We determined evidence for long-distance migration in four species (L. carinenta, E. claudia, D. glippus, Z. cesonia) and present evidence for panmixia (Z. cesonia), chain (Libytheana Citation: Hobson, K.A.; Kusack, J.W.; Mora-Alvarez, B.X. Origins of Six carinenta), and leapfrog (Danaus gilippus) migrations in three species. Our investigation underlines Species of Butterflies Migrating the utility of the stable isotope approach to quickly establish migratory origins and connectivity in through Northeastern Mexico: New butterflies and other insect taxa, especially if they can be sampled at migratory bottlenecks. -

Arthropods of Elm Fork Preserve
Arthropods of Elm Fork Preserve Arthropods are characterized by having jointed limbs and exoskeletons. They include a diverse assortment of creatures: Insects, spiders, crustaceans (crayfish, crabs, pill bugs), centipedes and millipedes among others. Column Headings Scientific Name: The phenomenal diversity of arthropods, creates numerous difficulties in the determination of species. Positive identification is often achieved only by specialists using obscure monographs to ‘key out’ a species by examining microscopic differences in anatomy. For our purposes in this survey of the fauna, classification at a lower level of resolution still yields valuable information. For instance, knowing that ant lions belong to the Family, Myrmeleontidae, allows us to quickly look them up on the Internet and be confident we are not being fooled by a common name that may also apply to some other, unrelated something. With the Family name firmly in hand, we may explore the natural history of ant lions without needing to know exactly which species we are viewing. In some instances identification is only readily available at an even higher ranking such as Class. Millipedes are in the Class Diplopoda. There are many Orders (O) of millipedes and they are not easily differentiated so this entry is best left at the rank of Class. A great deal of taxonomic reorganization has been occurring lately with advances in DNA analysis pointing out underlying connections and differences that were previously unrealized. For this reason, all other rankings aside from Family, Genus and Species have been omitted from the interior of the tables since many of these ranks are in a state of flux. -
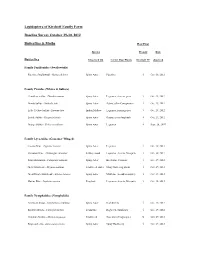
Butterflies and Moths List
Lepidoptera of Kirchoff Family Farm Baseline Survey October 29-30, 2012 Butterflies & Moths Host Plant Species Present Date Butterflies Observed On Larval Host Plants Kirchoff FF observed Family Papilionidae (Swallowtails) Pipevine Swallowtail - Battus philenor Spiny Aster Pipevine √ Oct. 30, 2012 Family Pieridae (Whites & Sulfurs) Cloudless Sulfur - Phoebis sennae Spiny Aster Legumes, clovers, peas √ Oct. 29, 2012 Dainty Sulfur - Nathalis iole Spiny Aster Asters, other Com;posites √ Oct. 29, 2012 Little Yellow Sulfur - Eurema lisa Indian Mallow Legumes, partridge pea √ Oct. 29, 2012 Lyside Sulfur - Kirgonia lyside Spiny Aster Guayacan or Soapbush √ Oct. 29, 2012 Orange Sulfur - Colias eurytheme Spiny Aster Legumes √ Sept. 26, 2007 Family Lycaenidae (Gossamer Winged) Cassius Blue - Leptotes cassius Spiny Aster Legumes √ Oct. 30, 2012 Ceraunus Blue - Hemiargus ceraunus Kidney wood Legumes- Acacia, Mesquite √ Oct. 30, 2012 Fatal Metalmark - Calephelis nemesis Spiny Aster Baccharis, Clematis √ Oct. 29, 2012 Gray Hairstreak - Strymon melinus Frostweed, Aster Many flowering plants √ Oct. 29, 2012 Great Purple Hairstreak - Atlides halesus Spiny Aster Mistletoe (in oak/mesquite) √ Oct. 29, 2012 Marine Blue - Leptotes marina Frogfruit Legumes- Acacia, Mesquite √ Oct. 30, 2012 Family Nymphalidae (Nymphalids) American Snout - Libytheana carinenta Spiny Aster Hackberries √ Oct. 29, 2012 Bordered Patch - Chlosyne lacinia Zexmenia Ragweed, Sunflower √ Oct. 29, 2012 Common Mestra - Mestra amymone Frostweed Noseburn (Tragia spp.) X Oct. 29, 2012 Empress Leila - Asterocampa leilia Spiny Aster Spiny Hackberry √ Oct. 29, 2012 Gulf Fritillary - Agraulis vanillae Spiny Aster Passion Vine X Oct. 29, 2012 Hackberry Emperor - Asterocampa celtis spiny Hackberry Hackberries √ Oct. 29, 2012 Painted Lady - Vanessa cardui Pink Smartweed Mallows & Thistles √ Oct. 29, 2012 Pearl Crescent - Phycoides tharos Spiny Aster Asters √ Oct. -

SYSTEMATICS of VAGRANTINI BUTTERFLIES (LEPIDOPTERA: Nymphalidae)
Treubia 2003 33 (1) 71-87 SYSTEMATICS OF VAGRANTINI BUTTERFLIES (LEPIDOPTERA: NYMPHAlIDAE). PART 1. CLADISTIC ANALYSIS Djunijanti Peggie . Division of Zoology, Research Center for Biology, Indonesian Institute of Sciences JI. Raya Jakarta Bogor Km. 46, Cibinong 16911, Indonesia Abstract Eiglit ge/lera of lndo-Australian butterjiies: Algia. Algiachroa, Cirrochroa, Cupha, Phalanta, Terinos, Vagrans, and Vindula are presented here. These genera together with two Afrotropical genera: Lachnoptera and Smerina, and a Central American genlls Euptoieta were previollsly placed as subiribe uncertain. One-hundred adult morphological characters were scored for fifty-four taxa, and were analyzed simultaneousuj (Nixon and Carpenter, 1993). The cladistic analysis showed that all species were properly assigned to monophyletic genera, and the arrangement of the outgroup taxa is in concordance with the classification previously suggested. The eight lndo-Australian and two Afrotropical genera belong to the tribe Vagrantini within the subfamily Heliconiinae. Key words: Heliconiines, Vagrantini, Indo-Australian, butterflies. Introduction The subfamily Heliconiinae is recognized by most authorities but the included taxa may differ. Ackery (in Vane-Wright and Ackery, 1984) suggested that the heliconiines may prove to represent a highly specialized subgroup of the Argynnini sensu lato. Heliconiinae sensu Harvey (in Nijhout, 1991) also include Acraeinae and Argynninae of Ackery (1988).Parsons (1999)included argynnines within Heliconiinae but retained Acraeinae as a distinct subfamily. Harvey (in N ijhou t, 1991) recognized three tribes of Heliconiinae: Pardopsini, Acraeini, and Heliconiini. The Heliconiini include the Neotropical Heliconiina (Brower, 2000), some genera which were placed as "subtribe uncertain", Argynnina, Boloriina and three other genera (the Neotropical genusYramea, the Oriental Kuekenthaliella, and Prokuekenthaliella) with uncertain relationships. -

Appendix a Sarah Tanedo/USFWS Common Tern with Chicks
Appendix A Sarah Tanedo/USFWS Common tern with chicks Animal Species Known or Suspected on Monomoy National Wildlife Refuge Table of Contents Table A.1. Fish Species Known or Suspected at Monomoy National Wildlife Refuge (NWR). ..........A-1 Table A.2. Reptile Species Known or Suspected on Monomoy NWR ............................A-8 Table A.3. Amphibian Species Known or Suspected on Monomoy NWR .........................A-8 Table A.4. Bird Species Known or Suspected on Monomoy NWR ..............................A-9 Table A.5. Mammal Species Known or Suspected on Monomoy NWR.......................... A-27 Table A.6. Butterfly and Moth Species Known or Suspected on Monomoy NWR. .................. A-30 Table A.7. Dragonfly and Damselfly Species Known or Suspected on Monomoy NWR. ............. A-31 Table A.8. Tiger Beetle Species Known or Suspected on Monomoy NWR ....................... A-32 Table A.9. Crustacean Species Known or Suspected on Monomoy NWR. ....................... A-33 Table A.10. Bivalve Species Known or Suspected on Monomoy NWR. ......................... A-34 Table A.11. Miscellaneous Marine Invertebrate Species at Monomoy NWR. .................... A-35 Table A.12. Miscellaneous Terrestrial Invertebrates Known to be Present on Monomoy NWR. ........ A-37 Table A.13. Marine Worms Known or Suspected at Monomoy NWR. .......................... A-37 Literature Cited ................................................................ A-40 Animal Species Known or Suspected on Monomoy National Wildlife Refuge Table A.1. Fish Species Known or Suspected at Monomoy National Wildlife Refuge (NWR). 5 15 4 1 1 2 3 6 6 Common Name Scientific Name Fall (%) (%) Rank Rank NOAA Spring Status Status Listing Federal Fisheries MA Legal Species MA Rarity AFS Status Occurrence Occurrence NALCC Rep. -

Ecology, Population Biology and Mortality of Euptoieta Hegesia Cramer (Nymphalidae) on Jamaica
}<mrnal <1 the Lepidopterists' Society 52(1), 1998, 9-39 ECOLOGY, POPULATION BIOLOGY AND MORTALITY OF EUPTOIETA HEGESIA CRAMER (NYMPHALIDAE) ON JAMAICA PHILLIP J. SCHAPPERT] AND JOEL S. SHORE Department of Biology, York University, 4700 Keele Street, North York, Ontario M3J IP3, Canada ABSTRACT. We examine the ecology, population biology and potential sources of mortality of Euptoieta hegesia, a tropical lowland butterfly from Jamaica, using a combina tion of captive rearing, studies of natural populations, and experimental approaches. We provide detailed observations of the life cycle and methods for captive rearing of this spe cies. We assess the relative performance of larvae on primary and secondary hostplants, distribution of larvae on the primary hostplant, hostplant population utilization, and the distribution of E. hegesia on the island. A mark-release-recapture study was conducted to estimate population parameters and we recorded sex, size, age (as estimated by wing wear), and wing damage sustained by the butterflies prior to their initial capture. We pro vide evidence that Tumera ulmifdia is the plimary hostplant of K hegesia on Jamaica and that butterfly population size is not limited by the availability of hostplants. These short lived butterflies appear to be residents of discrete hostplant populations and experience high mortality levels. Females are damaged more frequently, show more total damage and more frequent symmetrical hindwing damage (attributablc to ground-based predators) than do males. Wc compare the results of the population study with available studies of other tropical butterflies and suggest that lowland butterfly population structure and dy namics are Significantly different from that of rainforest species. -

Lepidoptera: Nymphalidae) THOMAS J
Morphology, molecules and fritillaries: approaching a stable phylogeny for Argynnini (Lepidoptera: Nymphalidae) THOMAS J. SIMONSEN, NIKLAS WAHLBERG, ANDREW V. Z. BROWER and RIENK de JONG Insect Syst.Evol. Simonsen, T. J., Wahlberg, N. , Brower, A. V. Z. & de Jong, R.: Morphology, molecules and fritillaries: approaching a stable phylogeny for Argynnini (Lepidoptera: Nymphalidae). Insect Syst. Evol. 37: 405-418. Copenhagen, December, 2006. ISSN 1399-560X. We examine the phylogenetic relationships among 29 species of Argynnini based on 141 pre- viously published morphological characters and new data from the mitochondrial gene COI and the two nuclear genes EF-1a and wingless. We investigate the stability and robustness of the resulting phylogenetic hypotheses through various combinations of the 4 functionally sep- arate datasets. Increasing the number of datasets in combined analyses led to increased sup- port for clades, sometimes substantially. We find that the tribe Argynnini is a well-supported, robust, monophyletic clade with the following internal subtribal structure: (Euptoietina (Yrameina (Boloriina Argynnina))). Our analyses support the classification of argynnine species into six robust and stable genera: Euptoieta, Yramea, Boloria, Issoria, Brenthis and Argynnis. We suggest that for moderate amounts of data, a total evidence approach is always best. Thomas J. Simonsen, Department of Entomology, Zoological Museum, University of Copen- hagen, Universitetsparken 15, DK-2100 Copenhagen, Denmark* Niklas Wahlberg, Department of Zoology, Stockholm University, S-106 91 Stockholm, Sweden Andrew V. Z. Brower, Department of Biology, Middle Tennessee State University, Murfrees- boro, TN 37132, USA Rienk de Jong, National Museum of Natural History, Department of Entomology, PO Box 9517, 2300 RA Leiden, The Netherlands *Corresponding author. -

Lepidoptera Are Relevant Bioindicators of Passive Regeneration in Tropical Dry Forests
diversity Article Lepidoptera are Relevant Bioindicators of Passive Regeneration in Tropical Dry Forests Luc Legal 1,* , Marine Valet 1, Oscar Dorado 2, Jose Maria de Jesus-Almonte 2, Karime López 2 and Régis Céréghino 1 1 Laboratoire écologie fonctionnelle et environnement, Université Paul Sabatier, CNRS, 31062 Toulouse, France; [email protected] (M.V.); [email protected] (R.C.) 2 Centro de Educación Ambiental e Investigación Sierra de Huautla, Universidad Autónoma del Estado de Morelos, Cuernavaca 62209, Mexico; [email protected] (O.D.); [email protected] (J.M.d.J.-A.); [email protected] (K.L.) * Correspondence: [email protected] Received: 12 April 2020; Accepted: 4 June 2020; Published: 9 June 2020 Abstract: Most evaluations of passive regeneration/natural succession or restoration have dealt with tropical rain forest or temperate ecosystems. Very few studies have examined the regeneration of tropical dry forests (TDF), one of the most damaged ecosystem types in the world. Owing to their species diversity and abundance, insects have been widely used as bioindicators of restoration. Butterflies were among the most abundant and useful groups. We sampled four sites with different levels of anthropogenic disturbance in a Mexican TDF (Morelos State) and compared butterfly communities. A first goal was to examine whether adult butterflies were significant bioindicators owing to their specificity to restricted habitats. A second aim was to determine if differences exist in butterfly communities between some fields abandoned from 4–8, 8–15 and 15–30 years and a reference zone considered as primary forest. We found 40% to 50% of the species of butterflies were specifically related to a habitat and/or a level of anthropogenic disturbance. -

The History and True Identity of Melitaea Ismeria (Nymphalidae): a Remarkable Tale of Duplication, Misinterpretation, and Presumption
Journal of the Lepidopterists' Society 57(3).2003, 204-219 THE HISTORY AND TRUE IDENTITY OF MELITAEA ISMERIA (NYMPHALIDAE): A REMARKABLE TALE OF DUPLICATION, MISINTERPRETATION, AND PRESUMPTION JOH:'-I V CALHOUN] 977 Wicks Drive, Palm Harbor, Florida 34684-4656, USA ABSTRACT. John Abbot (1751--<:a. 1840) supplied the watercolor drawing for the OIiginal description and accompanying engraved plate of Melitaea ismeria Boisduval and J ,e Conte. The plate was poorly executed, resulting in 170 years of debate regarding the identity of the fig ured species. ,\10st authors treated M. ismeria as synonymous with Chlosyne gorgone (Hiibner). More recently, a neotype of M. iS1'IWria was des ignated to reflect synonymy with Chlosyne nycteis (Doubleday), resnlting in a proposed priority replacement of nycteis. During a study to eval uate these findings, the original drawing of M. iS1'IW ria was discovered. John Abbot copied this drawing fmm an earlier painting of C. gorgone. Two other duplicates of this C. gorgone illustration were also located. The figured early stages and hostplant are consistent with C. gorgone. The proposed priority replacement ofnycteis is therefor", unwarranted. Also included are details about the drawings used by Boisduval and Le Conte and the discovery of a specimen of C. gorgone attlibuted to John Abbot. Additional key words: Chlosljne, Georgia, John Abbot, larva, pupa, South Carolina. Klots (1951) considered Melitaea ismeria Boisduval of Agriculture and Consumer Services, Gainesville), & Le Conte, [1833J to be "one of our greatest prob Houghton Library (Harvard University), Macleay Mu lems." Miller & Brown (1981) called it "a nomenclat seum (University of Sydney, New South Wales, Aus ural headache." Due to a poorly engraved illustration tralia ), The Natural History Museum, London that accompanied the original description, M. -

The Journal of Research on the Lepidoptera Volume 41 2002 (2009) the Journal of Research on the Lepidoptera
The Journal of Research on the Lepidoptera Volume 41 2002 (2009) Volume Lepidoptera the on Research of Journal The The Journal of Research on the Lepidoptera Volume 41 2002 (2009) IN THIS ISSUE Date of publication: January 31, 2009 PAPERS The life history of Hypanartia dione dione (Lepidoptera: Nymphalidae) in northeastern Ecuador Harold F. Greeney and Carina Chicaiza Aguirre 1 Updated phylogeny, taxonomy, and diversification ofJanthecla Robbins & Venables (Lycaenidae: Theclinae: Eumaeini) Robert K. Robbins and Robert C. Busby 5 δ 15N analyses of butterfly wings and bodies suggest minimal nitrogen absorption in carrion and dung puddling butterflies (Lepidoptera: Nymphalidae) Freerk Molleman and Jeremy Midgley 14 Cost-effectiveness of Philippine butterfly species used in live exhibits: an assessment of longevity, encounter rate and behaviour. Laura J. Robson, Adrienne Brewster and Gard Otis 17 The Neo-Riparian butterfly fauna of western Argentina Arthur M. Shapiro 24 Revisiting the pre-European butterfly fauna of the Sacramento Valley, California Arthur M. Shapiro 31 Population biology of Euptoieta hegesia (Nymphalidae: Heliconiinae: Argynnini) in an urban area in Southeastern Brazil Julia Losada Tourinho and André Victor Lucci Freitas 40 Observations of overwintering nymphalid butterflies in underground shelters in SW and W Bohemia (Czech Republic) (Lepidoptera: Nymphalidae: Nymphalini) Libor Dvoŕák, Joseph Belicek and Zdenĕk Fric 45 A revised classification scheme for larval hesperiid shelters, with comments on shelter diversity in the Pyrginae Harold F. Greeney 53 Preliminary field survey of butterflies on Xishan Hill (Kunming, Yunnan Province, China) Hu Shaoji 60 A newly observed form of symbiotic relationship between Reverdin’s blue Lycaeides argyrognomon praeterinsularis (Verity), (Lycaenidae) and Camponotus japonicus Mayr (Formicidae) Michihito Watanabe and Yasuo Hagiwara 70 NOTES Fabaceae, a new host plant family for Hypanartia and for the Neotropical Nymphalinae (Lepidoptera: Nymphalidae) Lucas A. -

A Preliminary Investigation of the Arthropod Fauna of Quitobaquito Springs Area, Organ Pipe Cactus National Monument, Arizona
COOPERATIVE NATIONAL PARK RESOURCES STUDIES UNIT UNIVERSITY OF ARIZONA 125 Biological Sciences (East) Bldg. 43 Tucson, Arizona 85721 R. Roy Johnson, Unit Leader National Park Senior Research Scientist TECHNICAL REPORT NO. 23 A PRELIMINARY INVESTIGATION OF THE ARTHROPOD FAUNA OF QUITOBAQUITO SPRINGS AREA, ORGAN PIPE CACTUS NATIONAL MONUMENT, ARIZONA KENNETH J. KINGSLEY, RICHARD A. BAILOWITZ, and ROBERT L. SMITH July 1987 NATIONAL PARK SERVICE/UNIVERSITY OF ARIZONA National Park Service Project Funds CONTRIBUTION NUMBER CPSU/UA 057/01 TABLE OF CONTENTS Introduction......................................................................................................................................1 Methods............................................................................................................................................1 Results ............................................................................................................................................2 Discussion......................................................................................................................................20 Literature Cited ..............................................................................................................................22 Acknowledgements........................................................................................................................23 LIST OF TABLES Table 1. Insects Collected at Quitobaquito Springs ...................................................................3