Endothelial Lipase Modulates Paraoxonase 1 Content and Arylesterase Activity of HDL
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Gentaur Products List
Chapter 2 : Gentaur Products List • Rabbit Anti LAMR1 Polyclonal Antibody Cy5 Conjugated Conjugated • Rabbit Anti Podoplanin gp36 Polyclonal Antibody Cy5 • Rabbit Anti LAMR1 CT Polyclonal Antibody Cy5 • Rabbit Anti phospho NFKB p65 Ser536 Polyclonal Conjugated Conjugated Antibody Cy5 Conjugated • Rabbit Anti CHRNA7 Polyclonal Antibody Cy5 Conjugated • Rat Anti IAA Monoclonal Antibody Cy5 Conjugated • Rabbit Anti EV71 VP1 CT Polyclonal Antibody Cy5 • Rabbit Anti Connexin 40 Polyclonal Antibody Cy5 • Rabbit Anti IAA Indole 3 Acetic Acid Polyclonal Antibody Conjugated Conjugated Cy5 Conjugated • Rabbit Anti LHR CGR Polyclonal Antibody Cy5 Conjugated • Rabbit Anti Integrin beta 7 Polyclonal Antibody Cy5 • Rabbit Anti Natrexone Polyclonal Antibody Cy5 Conjugated • Rabbit Anti MMP 20 Polyclonal Antibody Cy5 Conjugated Conjugated • Rabbit Anti Melamine Polyclonal Antibody Cy5 Conjugated • Rabbit Anti BCHE NT Polyclonal Antibody Cy5 Conjugated • Rabbit Anti NAP1 NAP1L1 Polyclonal Antibody Cy5 • Rabbit Anti Acetyl p53 K382 Polyclonal Antibody Cy5 • Rabbit Anti BCHE CT Polyclonal Antibody Cy5 Conjugated Conjugated Conjugated • Rabbit Anti HPV16 E6 Polyclonal Antibody Cy5 Conjugated • Rabbit Anti CCP Polyclonal Antibody Cy5 Conjugated • Rabbit Anti JAK2 Polyclonal Antibody Cy5 Conjugated • Rabbit Anti HPV18 E6 Polyclonal Antibody Cy5 Conjugated • Rabbit Anti HDC Polyclonal Antibody Cy5 Conjugated • Rabbit Anti Microsporidia protien Polyclonal Antibody Cy5 • Rabbit Anti HPV16 E7 Polyclonal Antibody Cy5 Conjugated • Rabbit Anti Neurocan Polyclonal -

Screening and Functional Pathway Analysis of Pulmonary Genes
Hindawi Stem Cells International Volume 2020, Article ID 5684250, 11 pages https://doi.org/10.1155/2020/5684250 Research Article Screening and Functional Pathway Analysis of Pulmonary Genes Associated with Suppression of Allergic Airway Inflammation by Adipose Stem Cell-Derived Extracellular Vesicles Sung-Dong Kim,1 Shin Ae Kang,2 Yong-Wan Kim,3 Hak Sun Yu,2 Kyu-Sup Cho ,1 and Hwan-Jung Roh 4 1Department of Otorhinolaryngology and Biomedical Research Institute, Pusan National University Hospital, Busan, Republic of Korea 2Department of Parasitology and Tropical Medicine, Pusan National University School of Medicine, Yangsan, Republic of Korea 3Department of Otorhinolaryngology, Inje University Haeundae Paik Hospital, Republic of Korea 4Department of Otorhinolaryngology and Research Institute for Convergence of Biomedical Science and Technology, Pusan National University Yangsan Hospital, Yangsan, Republic of Korea Correspondence should be addressed to Hwan-Jung Roh; [email protected] Received 5 February 2020; Revised 19 May 2020; Accepted 2 June 2020; Published 27 June 2020 Academic Editor: Kar Wey Yong Copyright © 2020 Sung-Dong Kim et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Background. Although mesenchymal stem cell- (MSC-) derived extracellular vesicles (EVs) are as effective as MSCs in the suppression of allergic airway inflammation, few studies have explored the molecular mechanisms of MSC-derived EVs in allergic airway diseases. The objective of this study was to evaluate differentially expressed genes (DEGs) in the lung associated with the suppression of allergic airway inflammation using adipose stem cell- (ASC-) derived EVs. -

Association of Paraoxonase-2 Genetic Variation with Serum
ASSOCIATION OF PARAOXONASE-2 GENETIC VARIATION WITH SERUM PARAOXONASE ACTIVITY AND SYSTEMIC LUPUS ERYTHEMATOSUS by Sudeshna Dasgupta B.S., University of Calcutta, India, 2001 M.S., University of Calcutta, India, 2003 Submitted to the Graduate Faculty of Graduate School of Public Health in partial fulfillment of the requirements for the degree of Doctor of Philosophy University of Pittsburgh 2008 UNIVERSITY OF PITTSBURGH Graduate School of Public Health This dissertation was presented by Sudeshna Dasgupta It was defended on November 3rd, 2008 and approved by F. Yesim Demirci M.D., Research Assistant Professor, Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh Susan M. Manzi, MD, MPH, Associate Professor, Department of Medicine, School of Medicine and Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh Candace M. Kammerer, Ph.D. Associate Professor, Department of Human Genetics Graduate School of Public Health, University of Pittsburgh Committee Chair Person Robert E. Ferrell, Ph.D. Professor, Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh Dissertation Advisor, M. Ilyas Kamboh, Ph.D. Professor and Chair, Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh ii Dedicated to my mother Mrs. Susmita Dasgupta and my father Dr. Gautam Dasgupta iii Copyright © by Sudeshna Dasgupta 2008 iv M. Ilyas Kamboh PhD ASSOCIATION OF PARAOXONASE-2 GENETIC VARIATION WITH SERUM PARAOXONASE ACTIVITY AND SYSTEMIC LUPUS ERYTHEMATOSUS Sudeshna Dasgupta, PhD University of Pittsburgh, 2008 SLE, a severe autoimmune disease is of major public health relevance since it predominantly affects women at child bearing age and even though immunosuppressives have increased the life span of SLE patients, lack of absolute cure is still troubling. -

PON1) Polymorphisms with Osteoporotic Fracture Risk in Postmenopausal Korean Women
EXPERIMENTAL and MOLECULAR MEDICINE, Vol. 43, No. 2, 71-81, February 2011 Association of Paraoxonase 1 (PON1) polymorphisms with osteoporotic fracture risk in postmenopausal Korean women Beom-Jun Kim1,2*, Shin-Yoon Kim2,6*, PON1 to ascertain its contribution to osteoporotic frac- Yoon Shin Cho3, Bon-Jo Kim3, Bok-Ghee Han3, tures (OFs) and bone mineral density (BMD). We di- Eui Kyun Park2,4, Seung Hun Lee1,2, Ha Young Kim5, rectly sequenced the PON1 gene in 24 Korean in- Ghi Su Kim1,2, Jong-Young Lee3,7 dividuals and identified 26 sequence variants. A large population of Korean postmenopausal women (n = and Jung-Min Koh1,2,7 1,329) was then genotyped for eight selected PON1 polymorphisms. BMD at the lumbar spine and femoral 1Division of Endocrinology and Metabolism neck was measured using dual-energy X-ray ab- Asan Medical Center, University of Ulsan College of Medicine sorptiometry. Lateral thoracolumbar (T4-L4) radio- Seoul 138-736, Korea 2 graphs were obtained for vertebral fracture assess- Skeletal Diseases Genome Research Center Kyungpook National University Hospital ment, and the occurrence of non-vertebral fractures Daegu 700-412, Korea (i.e., wrist, hip, forearm, humerus, rib, and pelvis) was 3The Center for Genome Science examined using self-reported data. Multivariate analy- National Institute of Health ses showed that none of the polymorphisms was asso- Seoul 122-701, Korea ciated with BMD at either site. However, +5989A>G 4Department of Pathology and Regenerative Medicine and +26080T>C polymorphisms were significantly School of Dentistry, Kyungpook National University associated with non-vertebral and vertebral fractures, Daegu 700-412, Korea respectively, after adjustment for covariates. -

Ameliorating Oxidative Stress and Inflammation by Hesperidin And
Turk J Biochem 2019; 44(2): 207–217 Research Article Thoria Donia*, Samar Eldaly and Ehab M.M. Ali Ameliorating oxidative stress and inflammation by Hesperidin and vitamin E in doxorubicin induced cardiomyopathy Doxorubicin ile İndüklenmiş Kardiyomiyopatide Hesperidin ve E Vitamini ile Oksidatif Stres ve İnflamasyonun İyileştirilmesi https://doi.org/10.1515/tjb-2018-0156 Conclusion: HSP and VIT.E possess a protective effect Received May 7, 2018; accepted July 3, 2018; previously published against DOX-induced cardiomyopathy via inhibiting oxi- online September 5, 2018 dative stress, inflammation, and apoptosis. Abstract Keywords: Cardiomyopathy; Doxorubicin; Hesperidin; Vitamin E; Oxidative stress. Background: Doxorubicin (DOX) is a common chemother- apeutic drug. However, it causes cardiomyopathy which reduces its clinical use in human cancer therapy. Öz Objective: The purpose of our study was to assess the cardioprotective effect of hesperidin (HSP) and vitamin E Giriş: Doksorubisin (DOX) yaygın bir kemoterapötik ilaçtır. (VIT.E) against DOX-induced cardiomyopathy. Bununla birlikte, kardiyomiyopatiye neden olduğu için bu Material and methods: Seventy rats were allocated into durum ilaçın insan kanser tedavisinde klinik kullanımını seven groups: control, HSP (50 mg/kg, orally), VIT.E azaltır. (100 mg/kg orally), DOX [4 mg/kg, intraperitoneally (i.p.)], Amaç: Çalışmamızın amacı, DOX ile indüklenen kardiyo- DOX + HSP, DOX + VIT.E and DOX + HSP + VIT.E. miyopatiye karşı hesperidin (HSP) ve vitamin E’nin (VIT.E) Results: Our findings showed that serum lactate dehy- kardiyoprotektif etkisini değerlendirmektir. drogenase (LDH), creatine kinase (CK), myeloperoxidase Gereç ve Yöntemler: Yetmiş sıçan yedi gruba ayrıldı: (MPO), cardiac catalase and caspase activities as well kontrol, HSP (50 mg/kg, oral), VIT.E (100 mg/kg oral), DOX as cardiac malondialdehyde (MDA) and serum nitric [4 mg/kg, intraperitoneal (ip)], DOX + HSP, DOX + VIT.E ve oxide (NO) concentrations were reduced DOX + HSP or DOX + HSP + VIT.E. -

Serendipitous Discovery and X-Ray Structure of a Human Phosphate Binding Apolipoprotein
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Structure 14, 601–609, March 2006 ª2006 Elsevier Ltd All rights reserved DOI 10.1016/j.str.2005.12.012 Serendipitous Discovery and X-Ray Structure of a Human Phosphate Binding Apolipoprotein Renaud Morales,1 Anne Berna,2 Philippe Carpentier,1 is the only known transporter capable of binding phos- Carlos Contreras-Martel,1 Fre´de´rique Renault,3 phate ions in human plasma and may become a new Murielle Nicodeme,4 Marie-Laure Chesne-Seck,6 predictor of or a potential therapeutic agent for phos- Franc¸ois Bernier,2 Je´roˆ me Dupuy,1 phate-related diseases such as atherosclerosis. Christine Schaeffer,7 He´le`ne Diemer,7 Alain Van-Dorsselaer,7 Juan C. Fontecilla-Camps,1 Introduction Patrick Masson,3 Daniel Rochu,3 and Eric Chabriere3,5,* Both cholesterol and HDL (high-density lipoprotein) 1 Laboratoire de Cristallogene` se et Cristallographie levels are commonly used as risk predictors of cardio- des Prote´ ines vascular disease (Gordon and Rifkind, 1989; Amann Institut de Biologie Structurale JP EBEL et al., 2003). HDL protects against atherosclerosis 38027 Grenoble mainly through the reverse cholesterol transport pro- France cess in which excess cholesterol is transferred from 2 Institut de Biologie Mole´ culaire des Plantes du CNRS peripheral tissues to the liver by the ATP binding cas- Universite´ Louis Pasteur sette ABCA1 transporter, which is defective in Tangier 67083 Strasbourg Cedex disease (Brooks-Wilson et al., 1999). Although HDLs France have been extensively studied, their metabolic role 3 Unite´ d’Enzymologie has not been completely elucidated yet. -
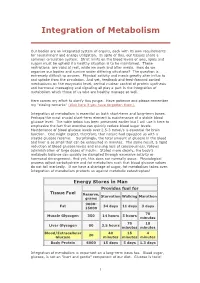
Integration of Metabolism
Integration of Metabolism Our bodies are an integrated system of organs, each with its own requirements for nourishment and energy utilization. In spite of this, our tissues share a common circulation system. Strict limits on the blood levels of ions, lipids and sugars must be upheld if a healthy situation is to be maintained. These restrictions are valid at rest, while we work and after meals. How do we organize our bodies and survive under differing situations? The question is extremely difficult to answer. Physical activity and meals greatly alter influx to and uptake from the circulation. And yet, feedback and feed-forward control mechanisms on the enzymatic level, central nuclear control of protein synthesis and hormonal messaging and signaling all play a part in the integration of metabolism which those of us who are healthy manage so well. Here comes my effort to clarify this jungle. Have patience and please remember my "closing remarks" (click here if you have forgotten them). Integration of metabolism is essential on both short-term and long-term bases. Perhaps the most crucial short-term element is maintenance of a stable blood glucose level. The table below has been presented earlier but I will use it here to emphasize the fact that exercise can quickly reduce blood sugar levels. Maintenance of blood glucose levels over 2.5-3 mmol/s is essential for brain function. One might expect, therefore, that nature had equipped us with a sizable glucose reserve. Surprisingly, the total amount of glucose in the blood and liver is so small that can be exhausted in minutes. -

The Metabolic Serine Hydrolases and Their Functions in Mammalian Physiology and Disease Jonathan Z
REVIEW pubs.acs.org/CR The Metabolic Serine Hydrolases and Their Functions in Mammalian Physiology and Disease Jonathan Z. Long* and Benjamin F. Cravatt* The Skaggs Institute for Chemical Biology and Department of Chemical Physiology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, California 92037, United States CONTENTS 2.4. Other Phospholipases 6034 1. Introduction 6023 2.4.1. LIPG (Endothelial Lipase) 6034 2. Small-Molecule Hydrolases 6023 2.4.2. PLA1A (Phosphatidylserine-Specific 2.1. Intracellular Neutral Lipases 6023 PLA1) 6035 2.1.1. LIPE (Hormone-Sensitive Lipase) 6024 2.4.3. LIPH and LIPI (Phosphatidic Acid-Specific 2.1.2. PNPLA2 (Adipose Triglyceride Lipase) 6024 PLA1R and β) 6035 2.1.3. MGLL (Monoacylglycerol Lipase) 6025 2.4.4. PLB1 (Phospholipase B) 6035 2.1.4. DAGLA and DAGLB (Diacylglycerol Lipase 2.4.5. DDHD1 and DDHD2 (DDHD Domain R and β) 6026 Containing 1 and 2) 6035 2.1.5. CES3 (Carboxylesterase 3) 6026 2.4.6. ABHD4 (Alpha/Beta Hydrolase Domain 2.1.6. AADACL1 (Arylacetamide Deacetylase-like 1) 6026 Containing 4) 6036 2.1.7. ABHD6 (Alpha/Beta Hydrolase Domain 2.5. Small-Molecule Amidases 6036 Containing 6) 6027 2.5.1. FAAH and FAAH2 (Fatty Acid Amide 2.1.8. ABHD12 (Alpha/Beta Hydrolase Domain Hydrolase and FAAH2) 6036 Containing 12) 6027 2.5.2. AFMID (Arylformamidase) 6037 2.2. Extracellular Neutral Lipases 6027 2.6. Acyl-CoA Hydrolases 6037 2.2.1. PNLIP (Pancreatic Lipase) 6028 2.6.1. FASN (Fatty Acid Synthase) 6037 2.2.2. PNLIPRP1 and PNLIPR2 (Pancreatic 2.6.2. -

Diagnostic Value of Serum Enzymes-A Review on Laboratory Investigations
Review Article ISSN 2250-0480 VOL 5/ ISSUE 4/OCT 2015 DIAGNOSTIC VALUE OF SERUM ENZYMES-A REVIEW ON LABORATORY INVESTIGATIONS. 1VIDYA SAGAR, M.SC., 2DR. VANDANA BERRY, MD AND DR.ROHIT J. CHAUDHARY, MD 1Vice Principal, Institute of Allied Health Sciences, Christian Medical College, Ludhiana 2Professor & Ex-Head of Microbiology Christian Medical College, Ludhiana 3Assistant Professor Department of Biochemistry Christian Medical College, Ludhiana ABSTRACT Enzymes are produced intracellularly, and released into the plasma and body fluids, where their activities can be measured by their abilities to accelerate the particular chemical reactions they catalyze. But different serum enzymes are raised when different tissues are damaged. So serum enzyme determination can be used both to detect cellular damage and to suggest its location in situ. Some of the biochemical markers such as alanine aminotransferase, aspartate aminotransferase, alkaline phasphatase, gamma glutamyl transferase, nucleotidase, ceruloplasmin, alpha fetoprotein, amylase, lipase, creatine phosphokinase and lactate dehydrogenase are mentioned to evaluate diseases of liver, pancreas, skeletal muscle, bone, etc. Such enzyme test may assist the physician in diagnosis and treatment. KEYWORDS: Liver Function tests, Serum Amylase, Lipase, CPK and LDH. INTRODUCTION mitochondrial AST is seen in extensive tissue necrosis during myocardial infarction and also in chronic Liver diseases like liver tissue degeneration DIAGNOSTIC SERUM ENZYME and necrosis². But lesser amounts are found in Enzymes are very helpful in the diagnosis of brain, pancreas and lung. Although GPT is plentiful cardiac, hepatic, pancreatic, muscular, skeltal and in the liver and occurs only in the small amount in malignant disorders. Serum for all enzyme tests the other tissues. -

GC-MS Metabolic Profile and -Glucosidase-, -Amylase-, Lipase-, and Acetylcholinesterase-Inhibitory Activities of Eight Peach
molecules Article GC-MS Metabolic Profile and α-Glucosidase-, α-Amylase-, Lipase-, and Acetylcholinesterase-Inhibitory Activities of Eight Peach Varieties Dasha Mihaylova 1,* , Ivelina Desseva 2,* , Aneta Popova 3 , Ivayla Dincheva 4 , Radka Vrancheva 2 , Anna Lante 5 and Albert Krastanov 1 1 Department of Biotechnology, Technological Faculty, University of Food Technologies, 4002 Plovdiv, Bulgaria; [email protected] 2 Department of Analytical Chemistry and Physical Chemistry, Technological Faculty, University of Food Technologies, 4002 Plovdiv, Bulgaria; [email protected] 3 Department of Catering and Tourism, Economics Faculty, University of Food Technologies, 4002 Plovdiv, Bulgaria; [email protected] 4 AgroBioInstitute, Agricultural Academy, 8 Dr. Tsankov Blvd., 1164 Sofia, Bulgaria; [email protected] 5 Department of Agronomy, Food, Natural Resources, Animals, and Environment—DAFNAE, Agripolis, University of Padova, 35020 Legnaro, Italy; [email protected] * Correspondence: [email protected] (D.M.); [email protected] (I.D.) Abstract: The inhibition of certain digestive enzymes by target food matrices represents a new approach in the treatment of socially significant diseases. Proving the ability of fruits to inhibit such enzymes can support the inclusion of specific varieties in the daily diets of patients with diabetes, obesity, Alzheimer’s disease, etc., providing them with much more than just valuable Citation: Mihaylova, D.; Desseva, I.; micro- and macromolecules. The current study aimed atidentifying and comparing the GC-MS Popova, A.; Dincheva, I.; Vrancheva, metabolic profiles of eight peach varieties (“Filina”, “Ufo 4, “Gergana”, “Laskava”, “July Lady”, R.; Lante, A.; Krastanov, A. GC-MS “Flat Queen”, “Evmolpiya”, and “Morsiani 90”) grown in Bulgaria (local and introduced) and to Metabolic Profile and α-Glucosidase-, evaluate the inhibitory potential of their extracts towards α-glucosidase, α-amylase, lipase, and α-Amylase-, Lipase-, and acetylcholinesterase. -

The Role of Phospholipase D (Pld) and Grb2 in Chemotaxis
THE ROLE OF PHOSPHOLIPASE D (PLD) AND GRB2 IN CHEMOTAXIS A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science By KATIE J. KNAPEK B.S., Indiana University of Pennyslvania, 2006 2008 Wright State University WRIGHT STATE UNIVERSITY SCHOOL OF GRADUATE STUDIES December 19, 2008 I HEREBY RECOMMEND THAT THE THESIS PREPARED UNDER MY SUPERVISON BY Katie J. Knapek ENTITLED The Role of Phospholipase D (PLD) and Grb2 in Chemotaxis BE ACCEPTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF Master of Science. _____________________________ Julian Gomez-Cambronero, Ph. D. Thesis Director _____________________________ Barbara Hull, Ph. D. Program Director Committee on Final Examination _______________________ Julian Gomez-Cambronero, Ph. D. _______________________ Nancy Bigley, Ph. D. _______________________ Mill Miller, Ph. D. ________________________ Joseph F. Thomas Jr., Ph. D. Dean, School of Graduate Studies ABSTRACT Knapek, Katie J. M.S., Department of Biological Sciences, Microbiology and Immunology Program, Wright State University, 2008. The Role of Phospholipase D (PLD) and Grb2 in Chemotaxis. Phospholipase D (PLD) is an enzyme that hydrolyzes phosphatidylcholine yielding choline and phosphatidic acid. PLD is activated by mitogens (lead to cell division) and motogens (leading to cell migration). PLD is known to contribute to cellular proliferation and deregulated expression of PLD has been implicated in several human cancers. PLD has been found to play a role in leukocyte chemotaxis and adhesion as studied through the formation of chemokine gradients. We have established a model of cell migration comprising three cell lines: macrophages RAW 264.7 and LR-5 (for innate defense), and fibroblast COS-7 cells (for wound healing). -

Multiple Roles of Phosphoinositide-Specific Phospholipase C Isozymes
BMB reports Mini Review Multiple roles of phosphoinositide-specific phospholipase C isozymes Pann-Ghill Suh1,*, Jae-Il Park1, Lucia Manzoli2, Lucio Cocco2, Joanna C. Peak3, Matilda Katan3, Kiyoko Fukami4, Tohru Kataoka5, Sanguk Yun1 & Sung Ho Ryu1 1Division of Molecular and Life Sciences, Pohang University of Science and Technology, Pohang, Korea, 2Cellular Signaling Laboratory, Department of Anatomical Sciences, University of Bologna, Via Irnerio, 48 I-40126, Bologna, Italy, 3Cancer Research UK Centre for Cell and Molecular Biology, Chester Beatty Laboratories, The Institute of Cancer Research, Fulham Road, London SW3 6JB, UK, 4Laboratory of Genome and Biosignal, Tokyo University of Pharmacy and Life Science, 1432-1 Horinouchi, Hachioji, 192-0392 Tokyo, Japan, 5Division of Molecular Biology, Department of Biochemistry and Molecular Biology, Kobe University Graduate School of Medicine, Chuo-ku, Kobe, Japan Phosphoinositide-specific phospholipase C is an effector mole- cellular calcium release (1-3; Fig. 1a). cule in the signal transduction process. It generates two sec- The first evidence of PLC activity was suggested by Hokin et ond messengers, inositol-1,4,5-trisphosphate and diacylglycer- al. in 1953 who reported specific hydrolysis of phospholipids ol from phosphatidylinositol 4,5-bisphosphate. Currently, thir- in pigeon’s pancreas slices after cholinergic stimulation (4). teen mammal PLC isozymes have been identified, and they are The authors showed that the enhanced turnover of phosphor- divided into six groups: PLC-β, -γ, -δ, -ε, -ζ and -η. Sequence ylinositol groups of phosphatidylinositol occurred in cells as a analysis studies demonstrated that each isozyme has more response to a variety of stimuli. In 1983, Streb et al.