Caveolin-1 Deficiency Impairs Synaptic Transmission in Hippocampal
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Clathrin-Independent Pathways of Endocytosis
Downloaded from http://cshperspectives.cshlp.org/ on October 3, 2021 - Published by Cold Spring Harbor Laboratory Press Clathrin-Independent Pathways of Endocytosis Satyajit Mayor1, Robert G. Parton2, and Julie G. Donaldson3 1National Centre for Biological Sciences, Tata Institute of Fundamental Research, and Institute for Stem Cell Biology and Regenerative Medicine, Bangalore 560065, India 2The University of Queensland, Institute for Molecular Bioscience and Centre for Microscopy and Microanalysis, Queensland 4072, Brisbane, Australia 3Cell Biology and Physiology Center, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, Maryland 20892 Correspondence: [email protected] There are many pathways of endocytosis at the cell surface that apparently operate at the same time. With the advent of new molecular genetic and imaging tools, an understanding of the different ways by which a cell may endocytose cargo is increasing by leaps and bounds. In this review we explore pathways of endocytosis that occur in the absence of clathrin. These are referred to as clathrin-independent endocytosis (CIE). Here we primarily focus on those pathways that function at the small scale in which some have distinct coats (caveolae) and others function in the absence of specific coated intermediates. We follow the trafficking itineraries of the material endocytosed by these pathways and finally discuss the functional roles that these pathways play in cell and tissue physiology. It is likely that these pathways will play key roles in the regulation of plasma membrane area and tension and also control the availability of membrane during cell migration. he identification of many of the components Consequently, CME has remained a pre- Tinvolved in clathrin-mediated endocytosis dominant paradigm for following the uptake (CME) and their subsequent characterization of material into the cell. -

Variable Priming of a Docked Synaptic Vesicle PNAS PLUS
Variable priming of a docked synaptic vesicle PNAS PLUS Jae Hoon Junga,b,c, Joseph A. Szulea,c, Robert M. Marshalla,c, and Uel J. McMahana,c,1 aDepartment of Neurobiology, Stanford University School of Medicine, Stanford, CA 94305; bDepartment of Physics, Stanford University School of Humanities and Sciences, Stanford, CA 94305; and cDepartment of Biology, Texas A&M University, College Station, TX 77845 Edited by Thomas S. Reese, National Institutes of Health, Bethesda, MD, and approved January 12, 2016 (received for review November 30, 2015) The priming of a docked synaptic vesicle determines the proba- transition. Biochemical and electrophysiological approaches have bility of its membrane (VM) fusing with the presynaptic membrane provided evidence that priming is mediated by interactions be- (PM) when a nerve impulse arrives. To gain insight into the nature tween the SNARE proteins and their regulators (7, 12–14, 24) and of priming, we searched by electron tomography for structural can involve differences in positioning of docked SVs relative to + relationships correlated with fusion probability at active zones of Ca2 channels (25). Biochemistry has also led to the suggestion axon terminals at frog neuromuscular junctions. For terminals that primed SVs may become deprimed (26). fixed at rest, the contact area between the VM of docked vesicles We have previously shown by electron tomography on frog and PM varied >10-fold with a normal distribution. There was no neuromuscular junctions (NMJs) fixed at rest that there are, for merging of the membranes. For terminals fixed during repetitive docked SVs, variations in the extent of the VM–PM contact area evoked synaptic transmission, the normal distribution of contact and in the length of the several AZM macromolecules linking areas was shifted to the left, due in part to a decreased number of the VM to the PM, the so-called ribs, pegs, and pins (2, 27). -

Mechanisms of Synaptic Plasticity Mediated by Clathrin Adaptor-Protein Complexes 1 and 2 in Mice
Mechanisms of synaptic plasticity mediated by Clathrin Adaptor-protein complexes 1 and 2 in mice Dissertation for the award of the degree “Doctor rerum naturalium” at the Georg-August-University Göttingen within the doctoral program “Molecular Biology of Cells” of the Georg-August University School of Science (GAUSS) Submitted by Ratnakar Mishra Born in Birpur, Bihar, India Göttingen, Germany 2019 1 Members of the Thesis Committee Prof. Dr. Peter Schu Institute for Cellular Biochemistry, (Supervisor and first referee) University Medical Center Göttingen, Germany Dr. Hans Dieter Schmitt Neurobiology, Max Planck Institute (Second referee) for Biophysical Chemistry, Göttingen, Germany Prof. Dr. med. Thomas A. Bayer Division of Molecular Psychiatry, University Medical Center, Göttingen, Germany Additional Members of the Examination Board Prof. Dr. Silvio O. Rizzoli Department of Neuro-and Sensory Physiology, University Medical Center Göttingen, Germany Dr. Roland Dosch Institute of Developmental Biochemistry, University Medical Center Göttingen, Germany Prof. Dr. med. Martin Oppermann Institute of Cellular and Molecular Immunology, University Medical Center, Göttingen, Germany Date of oral examination: 14th may 2019 2 Table of Contents List of abbreviations ................................................................................. 5 Abstract ................................................................................................... 7 Chapter 1: Introduction ............................................................................ -

Palmitoylation: Implications for Nitric Oxide Signaling
Proc. Natl. Acad. Sci. USA Vol. 93, pp. 6448-6453, June 1996 Cell Biology Targeting of nitric oxide synthase to endothelial cell caveolae via palmitoylation: Implications for nitric oxide signaling (endothelial nitric oxide synthase/signal transduction/vascular biology/N-myristoylation) GUILLERMO GARC1A-CARDENA*, PHIL OHt, JIANwEI LIu*, JAN E. SCHNITZERt, AND WILLIAM C. SESSA*t *Molecular Cardiobiology Program and Department of Pharmacology, Yale University School of Medicine, 295 Congress Avenue, New Haven, CT 06536; and tDepartment of Pathology, Harvard Medical School, Beth Israel Hospital, 330 Brookline Avenue, Boston, MA 02215 Communicated by Vincent T. Marchesi, Yale Univeristy, New Haven, CT, March 13, 1996 (received for review February 5, 1996) ABSTRACT The membrane association of endothelial insoluble membranes (TIM), suggesting that caveolae are nitric oxide synthase (eNOS) plays an important role in the signal processing centers (2-11). Additionally, caveolae have biosynthesis of nitric oxide (NO) in vascular endothelium. been implicated in other important cellular functions, includ- Previously, we have shown that in cultured endothelial cells ing endocytosis, potocytosis, and transcytosis (12, 13). and in intact blood vessels, eNOS is found primarily in the Endothelial nitric oxide synthase (eNOS) is a peripheral perinuclear region of the cells and in discrete regions of the membrane protein that metabolizes L-arginine to nitric oxide plasma membrane, suggesting trafficking of the protein from (NO). NO is a short-lived free radical gas involved in diverse the Golgi to specialized plasma membrane structures. Here, physiological and pathological processes. Endothelial-derived we show that eNOS is found in Triton X-100-insoluble mem- NO is an important paracrine mediator of vascular smooth branes prepared from cultured bovine aortic endothelial cells muscle tone and is an inhibitor of leukocyte adhesion and and colocalizes with caveolin, a coat protein of caveolae, in platelet aggregation (14, 15). -

Palmitoylation of Caveolin-1 and Its Importance for Structural and Functional Plasticity
Palmitoylation of Caveolin-1 and its importance for structural and functional plasticity A DISSERTATION SUBMITTED TO THE FACULTY OF UNIVERSITY OF MINNESOTA BY Katherine R. Tonn IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY Paul G. Mermelstein November 2018 © Katherine R. Tonn Eisinger 2018 Acknowledgments I would like to thank my adviser, Dr. Paul Mermelstein, for his mentorship, training, support, and scientific flexibility. Infinite thanks to past and present members of the Mermelstein and Meisel Labs, especially Dr. Brittni Peterson, Dr. Kelsey Moore, Dr. Laura Been, Dr. Luis Martinez, Dr. Valerie Hedges, and Dr. John Meitzen, for being a source of scientific inspiration and friendship. I could not have asked for a better friend and lab colleague than Dr. Kellie Gross, whose input has kept me sane and made me a better scientist. Thanks also to stellar undergrads Kerry Trotter, Julia Dworsky, and Sam Swanson for their help and energy. Thanks to the many people who collaborated or helped in some way on the experiments presented in this thesis, including Dr. Mark Thomas, Dr. Lorene Lanier, Dr. Mark Dell’Acqua, Dr. Kevin Woolfrey, Dr. Brian Head, Dr. Jing Tong, and Dr. John Meitzen. In particular, I am extremely grateful for the collaboration of Dr. Lorene Lanier, whose enthusiasm helped reinvigorate me and this project. Finally, thanks to the members of my thesis committee, Drs. Harry Orr, Timothy Ebner, Anna Lee, and Robert Meisel, for their encouragement and guidance. i Dedication For Mom, my first and most important example of what it is to be smart, versatile, and kind. -

Endothelial Plasmalemma Vesicle–Associated Protein Regulates the Homeostasis of Splenic Immature B Cells and B-1 B Cells
Endothelial Plasmalemma Vesicle−Associated Protein Regulates the Homeostasis of Splenic Immature B Cells and B-1 B Cells This information is current as Raul Elgueta, Dan Tse, Sophie J. Deharvengt, Marcus R. of September 26, 2021. Luciano, Catherine Carriere, Randolph J. Noelle and Radu V. Stan J Immunol 2016; 197:3970-3981; Prepublished online 14 October 2016; doi: 10.4049/jimmunol.1501859 Downloaded from http://www.jimmunol.org/content/197/10/3970 Supplementary http://www.jimmunol.org/content/suppl/2016/10/13/jimmunol.150185 Material 9.DCSupplemental http://www.jimmunol.org/ References This article cites 64 articles, 25 of which you can access for free at: http://www.jimmunol.org/content/197/10/3970.full#ref-list-1 Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision by guest on September 26, 2021 • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2016 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Endothelial Plasmalemma Vesicle–Associated Protein Regulates the Homeostasis of Splenic Immature B Cells and B-1 B Cells Raul Elgueta,*,† Dan Tse,‡,1 Sophie J. -

Part III: Modeling Neurotransmission – a Cholinergic Synapse
Part III: Modeling Neurotransmission – A Cholinergic Synapse Operation of the nervous system is dependent on the flow of information through chains of neurons functionally connected by synapses. The neuron conducting impulses toward the synapse is the presynaptic neuron, and the neuron transmitting the signal away from the synapse is the postsynaptic neuron. Chemical synapses are specialized for release and reception of chemical neurotransmitters. For the most part, neurotransmitter receptors in the membrane of the postsynaptic cell are either 1.) channel-linked receptors, which mediate fast synaptic transmission, or 2.) G protein-linked receptors, which oversee slow synaptic responses. Channel-linked receptors are ligand-gated ion channels that interact directly with a neurotransmitter and are called ionotropic receptors. Alternatively, metabotropic receptors do not have a channel that opens or closes but rather, are linked to a G-protein. Once the neurotransmitter binds to the metabotropic receptor, the receptor activates the G-protein which, in turn, goes on to activate another molecule. 3a. Model the ionotropic cholinergic synapse shown below. Be sure to label all of the following: voltage-gated sodium channel, voltage-gated potassium channel, neurotransmitter, synaptic vesicle, presynaptic cell, postsynaptic cell, potassium leak channel, sodium-potassium pump, synaptic cleft, acetylcholine receptor, acetylcholinesterase, calcium channel. When a nerve impulse (action potential) reaches the axon terminal, it sets into motion a chain of events that triggers the release of neurotransmitter. You will next model the events of neurotransmission at a cholinergic synapse. Cholinergic synapses utilize acetylcholine as the chemical of neurotransmission. MSOE Center for BioMolecular Modeling Synapse Kit: Section 3-6 | 1 Step 1 - Action potential arrives at the Step 2 - Calcium channels open in the terminal end of the presynaptic cell. -

Novel Missense Mutation in the Caveolin-3 Gene in a Belgian Family
1349 J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.2003.028217 on 16 August 2004. Downloaded from SHORT REPORT Novel missense mutation in the caveolin-3 gene in a Belgian family with rippling muscle disease P Y K Van den Bergh, J M Ge´rard, J A Elosegi, M U Manto, C Kubisch, B G H Schoser ............................................................................................................................... J Neurol Neurosurg Psychiatry 2004;75:1349–1351. doi: 10.1136/jnnp.2003.028217 too small’’). The patient was diagnosed as having fibromyal- Rippling muscle disease (RMD) is a rare muscle disorder gia, which led to secondary depression and loss of her job. characterised by muscle stiffness, exercise induced myalgia, Muscle weakness and pigmenturia were absent. The medical and cramp-like sensations. It is genetically heterogeneous history was remarkable for mild hypothyroidism, peptic and can be acquired, but most cases show autosomal oesophagitis, and tobacco related asthmatiform bronchitis dominant inheritance due to mutations in the caveolin-3 with emphysema. At age 38, she had a neurological work-up. (CAV3) gene. We report a novel heterozygous missense The cranial nerves, muscle bulk, muscle strength, and deep mutation in CAV3 in a Belgian family with autosomal tendon reflexes were normal, and plantar responses were dominant RMD. flexor. Tapping with the reflex hammer or with the finger on A 40 year old woman complained of fatigue, exercise the muscles provoked an immediate, short lasting, forceful induced muscle pain, and muscle cramps since the age of 35. contraction and/or painful local mounding lasting for several Neurological examination revealed percussion induced seconds. PIRCs were most pronounced in the sternocleido- rapid muscle contractions (PIRCs) and localised muscle mastoid, deltoid, biceps, brachioradialis, finger and wrist mounding on percussion; muscle rippling was not observed. -

Membrane Capacitance Recordings Resolve Dynamics and Complexity Of
www.nature.com/scientificreports OPEN Membrane capacitance recordings resolve dynamics and complexity of receptor-mediated endocytosis in Received: 22 October 2018 Accepted: 20 August 2019 Wnt signalling Published: xx xx xxxx Vera Bandmann1, Ann Schirin Mirsanaye1, Johanna Schäfer1, Gerhard Thiel1, Thomas Holstein2 & Melanie Mikosch-Wersching1,2 Receptor-mediated endocytosis is an essential process in signalling pathways for activation of intracellular signalling cascades. One example is the Wnt signalling pathway that seems to depend on endocytosis of the ligand-receptor complex for initiation of Wnt signal transduction. To date, the roles of diferent endocytic pathways in Wnt signalling, molecular players and the kinetics of the process remain unclear. Here, we monitored endocytosis in Wnt3a and Wnt5a-mediated signalling with membrane capacitance recordings of HEK293 cells. Our measurements revealed a swift and substantial increase in the number of endocytic vesicles. Extracellular Wnt ligands specifcally triggered endocytotic activity, which started immediately upon ligand binding and ceased within a period of ten minutes. By using specifc inhibitors, we were able to separate Wnt-induced endocytosis into two independent pathways. We demonstrate that canonical Wnt3a is taken up mainly by clathrin-independent endocytosis whereas noncanonical Wnt5a is exclusively regulated via clathrin-mediated endocytosis. Our fndings show that membrane capacitance recordings allow the resolution of complex cellular processes in plasma membrane signalling pathways in great detail. Wnt signalling is a highly-conserved signalling pathway with important functions in development, tissue-homeostasis, stem cell biology and many diseases, including cancer. Afer three decades of dedicated research we have come to understand many of the fundamental components of Wnt signalling pathways. -
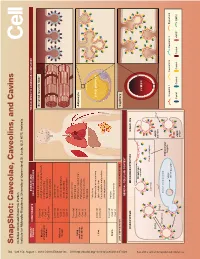
Snapshot: Caveolae, Caveolins, and Cavins Nicholas Ariotti and Robert G
704 Cell SnapShot: Caveolae, Caveolins, and Cavins Nicholas Ariotti and Robert G. Parton 154 Institute for Molecular Bioscience, University of Queensland, St. Lucia, QLD 4072, Australia , August 1,2013©2013 Elsevier Inc. DOI http://dx.doi.org/10.1016/j.cell.2013.07.009 ORGAN/ COMPONENTS DISEASE AND TISSUE-SPECIFIC CAVEOLAR COMPLEXES TISSUE CELLULAR PROCESS Rippling muscle disease Caveolin3 Striated muscle fiber Limb girdle muscular dystrophy Muscle Cavin1 skeletal and Cardiomyopathy Cavin4 cardiac Mechanoprotection Cav1 (heart) T-tubule formation Caveolin1 Lipodystrophy Caveolin2 Lipotoxicity Adipose Cavin1 Fatty acid regulation tissue Cavin2 Insulin signaling Cavin3 Mechanoprotection Atherosclerosis Caveolin1 Inammation Caveolin2 Lung Pulmonary hypertension and other Cavin1 Adipocyte Pulmonary brosis endothelia Cavin2 Mechanosensation Cavin3 Signaling Fatty liver Hepatocellular carcinoma Caveolin1 LIPID DROPLET Liver Lipid metabolism Caveolin2 Carbohydrate metabolism Liver regeneration Caveolin1 Autism Brain Cavin1 Schizophrenia Cavin3 LOW EXPRESSION HIGH EXPRESSION Capillary GENERAL CELLULAR CONTEXT ENDOCYTOSIS MECHANOPROTECTION SIGNALING See online version for legend and references. and legend for version online See Membrane stretch LUMEN Cavin complex Caveola EHD2 eNOS Ca2+ inactive Rab5 Dynamin2 EARLY ENDOSOME Intracellular targets MVB/ Caveolin 1 Caveolin 2 Caveolin 3 Dynamin2 late endosome eNOS Cavin1 Cavin2 Cavin3 Cavin4 eNOS EHD2 active SnapShot: Caveolae, Caveolins, and Cavins Nicholas Ariotti and Robert G. Parton Institute for Molecular Bioscience, University of Queensland, St. Lucia, QLD 4072, Australia Caveolae, submicroscopic bulb-shaped plasma membrane pits, are an abundant feature of many mammalian cells (Parton and del Pozo, 2013). Caveolae and the major proteins of caveolae, caveolins (Rothberg et al., 1992), and cavins (Hill et al., 2008), are linked to a number of human diseases such as muscular dystrophy, cardiomyopathy, and lipodys- trophy (see Glossary) (Bruno et al., 1993; Fernández et al., 2006; Hayashi et al., 2009). -

Molecular Mechanism of Fusion Pore Formation Driven by the Neuronal SNARE Complex
Molecular mechanism of fusion pore formation driven by the neuronal SNARE complex Satyan Sharmaa,1 and Manfred Lindaua,b aLaboratory for Nanoscale Cell Biology, Max Planck Institute for Biophysical Chemistry, 37077 Göttingen, Germany and bSchool of Applied and Engineering Physics, Cornell University, Ithaca, NY 14850 Edited by Axel T. Brunger, Stanford University, Stanford, CA, and approved November 1, 2018 (received for review October 2, 2018) Release of neurotransmitters from synaptic vesicles begins with a systems in which various copy numbers of syb2 were incorporated narrow fusion pore, the structure of which remains unresolved. To in an ND while the t-SNAREs were present on a liposome have obtain a structural model of the fusion pore, we performed coarse- been used experimentally to study SNARE-mediated mem- grained molecular dynamics simulations of fusion between a brane fusion (13, 17). The small dimensions of the ND compared nanodisc and a planar bilayer bridged by four partially unzipped with a spherical vesicle makes such systems ideally suited for MD SNARE complexes. The simulations revealed that zipping of SNARE simulations without introducing extreme curvature, which is well complexes pulls the polar C-terminal residues of the synaptobrevin known to strongly influence the propensity of fusion (18–20). 2 and syntaxin 1A transmembrane domains to form a hydrophilic MARTINI-based CGMD simulations have been used in several – core between the two distal leaflets, inducing fusion pore forma- studies of membrane fusion (16, 21 23). To elucidate the fusion tion. The estimated conductances of these fusion pores are in good pore structure and the mechanism of its formation, we performed agreement with experimental values. -

Comprehensive Analysis of Lncrna‑Associated Cerna Network Reveals the Novel Potential of Lncrna, Mirna and Mrna Biomarkers in Human Rectosigmoid Junction Cancer
ONCOLOGY LETTERS 21: 144, 2021 Comprehensive analysis of lncRNA‑associated ceRNA network reveals the novel potential of lncRNA, miRNA and mRNA biomarkers in human rectosigmoid junction cancer QIANSHI ZHANG1*, ZHEN FENG1*, SHASHA SHI2, YU ZHANG3 and SHUANGYI REN1 Departments of 1Gastrointestinal Surgery, 2Ultrasound and 3Clinical Laboratory, The Second Affiliated Hospital of Dalian Medical University, Dalian, Liaoning 116023, P.R. China Received March 11, 2020; Accepted November 20, 2020 DOI: 10.3892/ol.2020.12405 Abstract. Although accumulating evidence has confirmed gene 20) and three mRNAs (sodium‑ and chloride‑dependent the potential biological functions of long non‑coding RNAs taurine transporter, fibroblast growth factor 13 and tubulin (lncRNAs) as competitive endogenous RNAs (ceRNAs) in polyglutamylase TTLL7) were significantly associated with colorectal tumorigenesis and progression, few studies have OS (P<0.05). Additionally, two lncRNAs (KCNQ1OT1 focused on rectosigmoid junction cancer. In the present and MIR17HG) interacted with most of the DEmiRNAs. study, a comprehensive analysis was conducted to explore Notably, two top‑ranked miRNAs (hsa‑miR‑374a‑5p and lncRNA‑mediated ceRNA implications and their potential hsa‑miR‑374b‑5p) associated networks were identified to be value for prognosis. lncRNA, microRNA (miR/miRNA) markedly associated with the pathogenesis. Furthermore, four and mRNA expression profiles were downloaded from DEmRNAs (caveolin‑1, MET, filamin‑A and AKT3) were The Cancer Genome Atlas database. Subsequently, a enriched in the Kyoto Encylopedia of Gene and Genomes lncRNA‑miRNA‑mRNA regulatory network was constructed pathway analysis, as well as being included in the ceRNA to evaluate the functions of these differentially expressed genes network. In summary, the present results revealed that a on overall survival (OS) for rectosigmoid junction cancer.