Malformation Syndromes Caused by Disorders of Cholesterol Synthesis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Differential Regulation of Gene Expression by Cholesterol Biosynthesis Inhibitors That Reduce (Pravastatin) Or Enhance (Squalest
JPET Fast Forward. Published on May 25, 2016 as DOI: 10.1124/jpet.116.233312 This article has not been copyedited and formatted. The final version may differ from this version. Differential regulation of gene expression by cholesterol biosynthesis inhibitors that reduce (pravastatin) or enhance (squalestatin 1) nonsterol isoprenoid levels in primary cultured mouse and rat hepatocytes. Elizabeth A. Rondini, Zofia Duniec-Dmuchowski, Daniela Cukovic, Alan A. Dombkowski, and Thomas A. Kocarek Downloaded from Institute of Environmental Health Sciences, Wayne State University, Detroit, MI 48202, USA (E.A.R., Z.D-D, T.A.K.) jpet.aspetjournals.org Department of Pediatrics, Division of Clinical Pharmacology and Toxicology, Wayne State University, Detroit, MI 48202 (D.C., A.A.D) at ASPET Journals on September 27, 2021 JPET Fast Forward. Published on May 25, 2016 as DOI: 10.1124/jpet.116.233312 This article has not been copyedited and formatted. The final version may differ from this version. JPET #233312 Running title: Regulation of hepatocellular gene expression by isoprenoids Address correspondence to: Dr. Thomas A. Kocarek, Institute of Environmental Health Sciences, 6135 Woodward Avenue, IBio Building, Room 2126, Wayne State University, Detroit, MI 48202, USA. Tel: (313) 577-6580; FAX: (313) 972-8025; E-mail: [email protected] Number of text pages: 43 Downloaded from Number of tables: 2 Supplemental Number of figures: 8 jpet.aspetjournals.org Number of references: 77 Number of words in Abstract: 249 Number of words in Introduction: 745 at -

Identification and Characterization of Zebrafish 17Beta-HSD Type 1 and Type 3: a Comparative Analysis of Androgen/Estrogen Activity Regulators
Institut für Experimentelle Genetik GSF-Forschungzentrum für Umwelt und Gesundheit, Neuherberg Identification and characterization of zebrafish 17beta-HSD type 1 and type 3: A comparative analysis of androgen/estrogen activity regulators Rebekka Mindnich Vollständiger Abdruck der von der Fakultät Wissenschaftszentrum Weihenstephan für Ernährung, Landnutzung und Umwelt der Technischen Universität München zur Erlangung des akademischen Grades eines Doktors der Naturwissenschaften genehmigten Dissertation. Vorsitzender: Univ.- Prof. Dr. Bertold Hock Prüfer der Dissertation: 1. Priv.-Doz. Dr. Jerzy Adamski 2. Univ.-Prof. Dr. Johannes Buchner 3. Univ.-Prof. Dr. Wolfgang Wurst Die Dissertation wurde am 30.06.2004 bei der Technischen Universität München eingereicht und durch die Fakultät Wissenschaftszentrum Weihenstephan für Ernährung, Landnutzung und Umwelt am 07.10. 2004 angenommen. Table of contents Table of contents ABSTRACT................................................................................................................................... 7 ZUSAMMENFASSUNG................................................................................................................ 9 ABBREVIATIONS....................................................................................................................... 11 1 INTRODUCTION ................................................................................................................ 13 1.1 THE AIM OF THIS STUDY ............................................................................................... -
Case Report Full Text Online At
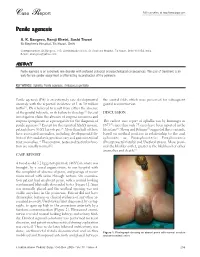
Case Report Full text online at http://www.jiaps.com Penile agenesis A. K. Bangroo, Ramji Khetri, Sashi Tiwari St Stephen's Hospital, Tis Hazari, Delhi Correspondence: AK Bangroo, 103, Administrative block, St. Stephens Hospital, Tis Hazari, Delhi-110054, India. E-mail: [email protected] ABSTRACT Penile agenesis is an extremely rare disorder with profound urological and psychological consequences. The goal of treatment is an early female gender assignment and feminizing reconstruction of the perineum. KEY WORDS: Aphallia, Penile agenesis, Ambiguous genitalia Penile agenesis (PA) is an extremely rare developmental the scrotal folds which were preserved for subsequent anomaly with the reported incidence of 1 in 30 million genital reconstruction. births[1]. PA is believed to result from either the absence of the genital tubercle, or its failure to develop.[2] Several DISCUSSION investigators claim the absence of corpora cavernosa and corpora spongiosum as a prerequisite for the diagnosis of The earliest case report of aphallia was by Imminger in penile agenesis.[3] Except for the reported XX-XY mosaic, 1853[2] since then only 75 cases have been reported in the patients have 46 XY karyotypes.[4] More than half of these literature[6]. Skoog and Belman[5] suggested three variants, have associated anomalies, including developmental de based on urethral position in relationship to the anal fects of the caudal axis, genitourinary and gastrointestinal sphincter, as: Postsphincteric; Presphincteric tract anomalies.[5] The scrotum, testes and testicular func (Prostatorectal fistula) and Urethral atresia. More proxi tion are usually normal[2]. mal the bladder outlet, greater is the likelihood of other anomalies and death.[5] CASE REPORT A two-day-old 3.2 kg genotypic male (46XY) neonate was brought, by a social organization, to our hospital with the complaint of absence of penis, and passage of meco nium mixed with urine through rectum. -

Characterization of the Ergosterol Biosynthesis Pathway in Ceratocystidaceae
Journal of Fungi Article Characterization of the Ergosterol Biosynthesis Pathway in Ceratocystidaceae Mohammad Sayari 1,2,*, Magrieta A. van der Nest 1,3, Emma T. Steenkamp 1, Saleh Rahimlou 4 , Almuth Hammerbacher 1 and Brenda D. Wingfield 1 1 Department of Biochemistry, Genetics and Microbiology, Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, Pretoria 0002, South Africa; [email protected] (M.A.v.d.N.); [email protected] (E.T.S.); [email protected] (A.H.); brenda.wingfi[email protected] (B.D.W.) 2 Department of Plant Science, University of Manitoba, 222 Agriculture Building, Winnipeg, MB R3T 2N2, Canada 3 Biotechnology Platform, Agricultural Research Council (ARC), Onderstepoort Campus, Pretoria 0110, South Africa 4 Department of Mycology and Microbiology, University of Tartu, 14A Ravila, 50411 Tartu, Estonia; [email protected] * Correspondence: [email protected]; Fax: +1-204-474-7528 Abstract: Terpenes represent the biggest group of natural compounds on earth. This large class of organic hydrocarbons is distributed among all cellular organisms, including fungi. The different classes of terpenes produced by fungi are mono, sesqui, di- and triterpenes, although triterpene ergosterol is the main sterol identified in cell membranes of these organisms. The availability of genomic data from members in the Ceratocystidaceae enabled the detection and characterization of the genes encoding the enzymes in the mevalonate and ergosterol biosynthetic pathways. Using Citation: Sayari, M.; van der Nest, a bioinformatics approach, fungal orthologs of sterol biosynthesis genes in nine different species M.A.; Steenkamp, E.T.; Rahimlou, S.; of the Ceratocystidaceae were identified. -

Experiences of Rare Diseases: an Insight from Patients and Families
Experiences of Rare Diseases: An Insight from Patients and Families Unit 4D, Leroy House 436 Essex Road London N1 3QP tel: 02077043141 fax: 02073591447 [email protected] www.raredisease.org.uk By Lauren Limb, Stephen Nutt and Alev Sen - December 2010 Web and press design www.raredisease.org.uk WordsAndPeople.com About Rare Disease UK Rare Disease UK (RDUK) is the national alliance for people with rare diseases and all who support them. Our membership is open to all and includes patient organisations, clinicians, researchers, academics, industry and individuals with an interest in rare diseases. RDUK was established by Genetic RDUK is campaigning for a Alliance UK, the national charity strategy for integrated service of over 130 patient organisations delivery for rare diseases. This supporting all those affected by would coordinate: genetic conditions, in conjunction with other key stakeholders | Research in November 2008 following the European Commission’s | Prevention and diagnosis Communication on Rare Diseases: | Treatment and care Europe’s Challenges. | Information Subsequently RDUK successfully | Commissioning and planning campaigned for the adoption of the Council of the European into one cohesive strategy for all Union’s Recommendation on patients affected by rare disease in an action in the field of rare the UK. As well as securing better diseases. The Recommendation outcomes for patients, a strategy was adopted unanimously by each would enable the most effective Member State of the EU (including use of NHS resources. the -

A MRI Diagnosis of Congenital Urogenital Anomalies in 27 Years
Journal of Advances in Radiology and Medical Imaging Volume 4 | Issue 1 ISSN: 2456-5504 Case Report Open Access A MRI Diagnosis of Congenital Urogenital Anomalies in 27 Years Old Man D’Amato D*, Ranalli T, Tatulli D, Bocchinfuso F, Manenti G, Valente F and Bizzaglia M Diagnostic and Interventional Radiology, Policlinico Tor Vergata, University of Rome “Tor Vergata”, Rome, Italy *Corresponding author: D’Amato D, Diagnostic and Interventional Radiology, Policlinico Tor Vergata, University of Rome “Tor Vergata”, Viale Oxford 181, Rome, Italy, Tel: +393207034690, E-mail: [email protected] Citation: D’Amato D, Ranalli T, Tatulli D, Bocchinfuso F, Manenti G, et al. (2019) A MRI Diagnosis of Congenital Urogenital Anomalies in 27 Years Old Man. J Adv Radiol Med Image 4(1): 102 Received Date: April 04, 2019 Accepted Date: August 26, 2019 Published Date: August 28, 2019 Abstract Congenital anorchia is an uncommon clinical condition. Etiology and pathogenetic mechanisms are often unknown. Although some patients with anorchia present with ambiguous external genitalia or micropenis, most have a normal phenotype. XY Disorders of Sex Development classifications are numerous and success rate in establishing a precise diagnosis is far lower than in XX karyotype. We report the case of a young man, with 46 XY karyotype showing various uro-genital abnormalities. A definitive diagnosis was not established due to the complex clinical presentation. Ultrasonography and Magnetic Resonance Imaging techniques were useful tools in the definition of uro-genital anomalies and gonadal development in this complex scenario. Keywords: Anorchia; Cryptorchidism; Urogenital Anomalies; DSD; MRI List of abbreviations: MRI: Magnetic Resonance Imaging; US: Ultrasonography; DSD: Disorders of Sex Development, FSH: Follicle- Stimulating Hormone; HCG: Human Chorionic Gonadotropin; LH: Luteinizing Hormone; AMH Antimüllerian Hormone; LHRH LH- Releasing Hormone; SD: Standard Deviation Introduction The disorders of sexual differentiation constitute a challenging area for both diagnostic and therapeutic impact. -

Inheritance of Multiple Epiphyseal Dysplasia, Tarda
Inheritance of Multiple Epiphyseal Dysplasia, Tarda RICHARD C. JUBERG1 AND JOHN F. HOLT2 Multiple epiphyseal dysplasia, tarda, a modeling error of the epiphyses, is charac- terized by shortness of stature and micromelia, particularly stubby hands (Rubin, 1964). A patient with this trait generally shows satisfactory development, adequate muscle mass, and normal intelligence. As a rule, there are few complaints of joint discomfort during childhood. Eventually, however, problems arise from joint motion, which lead to difficulty in walking because of pain in the hips, knees, or ankles. De- formities may result. On the other hand, the defect may not be recognized until the patient presents manifestations of degenerative arthritis at an unusually early age. The classification of epiphyseal modeling errors by Rubin (1964) contains six different entities. Spondyloepiphyseal dysplasia, congenita (Morquio's disease) is characterized by irregularity of epiphyses and vertebrae. It is an abnormality of mucopolysaccharide metabolism, and it is transmitted as an autosomal recessive. Multiple epiphyseal dysplasia, congenita (stippled epiphyses) is characterized by stippled or punctate epiphyses, and it too is transmitted as an autosomal recessive. Epiphyseal retardation, which occurs in cretinism (hypothyroidism), may result from any of several different metabolic errors which appear to be transmitted as autosomal recessives (Stanbury, 1966). Diastrophic dwarfism results in delayed appearance of epiphyses and joint luxations, and this, too, apparently is transmitted as an autosomal recessive. Multiple epiphyseal dysplasia, tarda, which is also known in the literature as epi- physeal dysplasia multiplex, must be differentiated from spondyloepiphyseal dys- plasia, tarda. As the name implies, one difference is in the extent and severity of spinal involvement as well as in the changes in the epiphyses of the long tubular bones. -

Estrogen Receptor-Mediated Neuroprotection: the Role of the Alzheimer’S Disease-Related Gene Seladin-1
REVIEW Estrogen receptor-mediated neuroprotection: The role of the Alzheimer’s disease-related gene seladin-1 Alessandro Peri Abstract: Experimental evidence supports a protective role of estrogen in the brain. According Mario Serio to the fact that Alzheimer’s disease (AD) is more common in postmenopausal women, estrogen treatment has been proposed. However, there is no general consensus on the benefi cial effect of Department of Clinical Physiopathology, Endocrine Unit, estrogen or selective estrogen receptor modulators in preventing or treating AD. It has to be said that Center for Research, Transfer several factors may markedly affect the effi cacy of the treatment. A few years ago, the seladin-1 gene and High Education on Chronic, Inflammatory, Degenerative (for selective Alzheimer’s disease indicator-1) has been isolated and found to be down-regulated and Neoplastic Disorders in brain regions affected by AD. Seladin-1 has been found to be identical to the gene encoding the for the Development of Novel enzyme 3-beta-hydroxysterol delta-24-reductase, involved in the cholesterol biosynthetic pathway, Therapies (DENOThe), University β of Florence, Florence, Italy which confers protection against -amyloid-mediated toxicity and from oxidative stress, and is an effective inhibitor of caspase-3 activity, a key mediator of apoptosis. Interestingly, we found earlier that the expression of this gene is up-regulated by estrogen. Furthermore, our very recent data support the hypothesis that seladin-1 is a mediator of the neuroprotective effects of estrogen. This review will summarize the current knowledge regarding the neuroprotective effects of seladin-1 and the relationship between this protein and estrogen. -

Opsoclonus-Myoclonus Syndrome
OMS Opsoclonus-Myoclonus Syndrome REGISTRY POWERED BY NORD 10 11 Tr io Health © 2019 Trio Health Advisory Group, Inc.; NORD - National Organization for Rare Disorders, Inc. | All rights reserved. © 2019 Trio Health Advisory Group, Inc.; NORD - National Organization for Rare Disorders, Inc. | All rights reserved. Tr io Health Meet OMS Warrior ALEXA What is OMS? OPSOCLONUS-MYOCLONUS SYNDROME General Discussion Opsoclonus-myoclonus syndrome (OMS) is an inflammatory neurological disorder, often occurring as a paraneoplastic syndrome with neurological symptoms being the first sign of an occult tumor. It is characterized by associated ocular, motor, behavioral, sleep, and language disturbances. The onset is oftentimes abrupt and can be relatively severe, with the potential to become chronic unless the appropriate diagnosis and treatment are reached in a timely manner. Signs and Symptoms The component features of OMS include the presence of rapid, seemingly random eye movements in the horizontal, vertical, and diagonal directions (opsoclonus); an unsteady gait or inability to walk or stand (ataxia); and brief, repetitive, shock-like muscle spasms or tremors within the arms, legs, or hands interfering with normal use (myoclonus). Behavioral and sleep disturbances, including extreme irritability, inconsolable crying, reduced or fragmented sleep (insomnia), and rage attacks are common. Difficulty articulating speech (dysarthria), sometimes with complete loss of speech and language, may occur. Additional symptoms, such as decreased muscle tone (hypotonia) and vomiting, have also been noted. Causes The most common cause of OMS in young children is an occult (ie, a small, often hidden) tumor. The symptoms of OMS, as a paraneoplastic syndrome, presumably stem from the immune system attacking the tumor, leading to secondary inflammatory effects on the central nervous system. -

DSD Population (Differences of Sex Development) in Barcelona BC N Area of Citizen Rights, Participation and Transparency
An analysis of the different realities, positions and requirements of the intersex / DSD population (differences of sex development) in Barcelona BC N Area of Citizen Rights, Participation and Transparency An analysis of the different realities, positions and requirements of the intersex / DSD population (differences of sex development) in Barcelona Barcelona, November 2016 This publication forms part of the deployment of the Municipal Plan for Sexual and Gender Diversity and LGTBI Equality Measures 2016 - 2020 Author of the study: Núria Gregori Flor, PhD in Social and Cultural Anthropology Proofreading and Translation: Tau Traduccions SL Graphic design: Kike Vergés We would like to thank all of the respond- ents who were interviewed and shared their knowledge and experiences with us, offering a deeper and more intricate look at the discourses and experiences of the intersex / Differences of Sex Develop- ment community. CONTENTS CHAPTER I 66 An introduction to this preliminary study .............................................................................................................. 7 The occurrence of intersex and different ways to approach it. Imposed and enforced categories .....................................................................................14 Existing definitions and classifications ....................................................................................................................... 14 Who does this study address? .................................................................................................................................................. -

A Defective Response to Hedgehog Signaling in Disorders of Cholesterol Biosynthesis
letter A defective response to Hedgehog signaling in disorders of cholesterol biosynthesis Michael K. Cooper1,2,5, Christopher A. Wassif3, Patrycja A. Krakowiak3, Jussi Taipale1, Ruoyu Gong1, Richard I. Kelley4, Forbes D. Porter3 & Philip A. Beachy1 Published online 24 March 2003; doi:10.1038/ng1134 Smith–Lemli–Opitz syndrome (SLOS), desmosterolosis and lath- additional role for cholesterol in Hh signal response was sug- osterolosis are human syndromes caused by defects in the final gested by the observation that cyclopamine and jervine, terato- stages of cholesterol biosynthesis. Many of the developmental genic plant alkaloids that block Hh signaling, also inhibit malformations in these syndromes occur in tissues and struc- cholesterol transport and synthesis2,3. But cyclopamine has since tures whose embryonic patterning depends on signaling by the been shown to specifically inhibit Hh signaling by binding to a Hedgehog (Hh) family of secreted proteins. Here we report that pathway component4, and the doses of these alkaloids required response to the Hh signal is compromised in mutant cells from to inhibit Hh signaling are lower than those required to block mouse models of SLOS and lathosterolosis and in normal cells cholesterol transport (ref. 5 and M.K.C., unpublished data). pharmacologically depleted of sterols. We show that decreas- To determine how cholesterol may affect Hh signaling in embry- ing levels of cellular sterols correlate with diminishing respon- onic development, we exposed chick embryos to cyclodextrin, a http://www.nature.com/naturegenetics siveness to the Hh signal. This diminished response occurs at cyclic oligosaccharide that forms non-covalent complexes with sterol levels sufficient for normal autoprocessing of Hh protein, sterols6 and can be used to extract and deplete cholesterol from liv- which requires cholesterol as cofactor and covalent adduct. -

Disorders of Sexual Differentiation and Surgical Corrections
Marmara Medical Journal Volume 2 No: 5 April 1989 DISORDERS OF SEXUAL DIFFERENTIATION AND SURGICAL CORRECTIONS C. Ôzsoy, M.D.* / A. KadioÇlu, M.D.*** / H. Ander, M.D.** * Professor, Department of Urology, Istanbul Medical Faculty, Istanbul, Turkey. ** Associate Professor, Department of Urology, Istanbul Medical Faculty, Istanbul, Turkey. * * * Research Assistant, Department of Urology, Istanbul Medical Faculty, Istanbul, Turkey. SUMMARY Patients with ambiguous genitalia who applied to our ?). Developments in Cytogenetics and Radio-immu- clinic are investigated and they are classified as true no assay after 1950's provided the easy diagnosis of hermaphroditism, male and female pseudohermaph sexual differentiation disorders. roditism. Normal sexual differentiation: The normal human After chromosomal, psychological, hormonal, phe diploid cell contains 22 autosomal pairs of chromoso notypic and surgical evaluation, the final sex is de mes and 2 sex chromosomes. Except spermatozoon termined and appropriate reconstructive surgery is and oocyt normal human cell is diploid. The chromo performed. somal sex is determined at the time of fertilization. An XX female or male determined chromosomal sex, In six of our 18 patients we reassigned the female sex influences sexual differentiation by causing the bipo- to male. 2 of our 18 patients are true hermaphrodites tenlial gonad to develop either as a testis or as an (one being male and the other is female). 2 of them are ovary. female pseudohermaphrodites and 14 of them are male pseudohermaphrodites. 6 patients who under Until the 7th week of gestation the gonads are indis went sexual reassignment were male pseudoher- tinguishable. In the presence of Y chromosome or H- maphrodiles. Y antigen, which is located on the short arm of X chromosome, the medulla of gonads will begin testi In our opinion the most important aspect of sex reas cular differentiation.