Contrasting Extreme Longdistance Migration Patterns in Bartailed
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The First Record of Far Eastern Curlew (Numenius Madagascariensis) in British Columbia
The First Record of Far Eastern Curlew (Numenius madagascariensis) in British Columbia. By Rick Toochin and Don Cecile. Submitted: April 15, 2018. Introduction and Distribution The Far Eastern Curlew (Numenius madagascariensis) is the largest migratory shorebird in the world. This species is found only along the East Asian–Australasian Flyway. The Far Eastern Curlew breeds on open mossy or transitional bogs, moss-lichen bogs and wet meadows, and on the swampy shores of small lakes in Siberia and Kamchatka in Russia, as well as in north-eastern Mongolia and China (Hayman et al. 1986, del Hoyo et al. 1996). The Yellow Sea of the Republic of Korea and China is a vitally important stopover site on migration. This species is also a common passage migrant in Japan and Indonesia, and is occasionally recorded moving through Thailand, Brunei, Bangladesh, Vietnam, Philippines, Malaysia and Singapore (O’Brien et al. 2006). During the winter a few birds occur in southern Republic of Korea, Japan, China, and Taiwan (Brazil 2009, EAAFP 2017). About 25% of the population is thought to winter in the Philippines, Indonesia and Papua New Guinea. Most birds, approximately 73% or 28,000 individuals, spend the winter in Australia, where birds are found primarily on the coast of all states, particularly the north, east and south-east regions including Tasmania (Bamford et al. 2008, BirdLife 2016). In the early 2000’s, the global population of the Far Eastern Curlew was estimated at 38,000 individuals (BirdLife 2016). Unfortunately due to the fact that the global population is declining, the true population size is likely to be much smaller, and may not exceed 20,000 individuals (BirdLife 2016). -

Age-Related Differences in Ruddy Turnstone Foraging and Aggressive Behavior
AGE-RELATED DIFFERENCES IN RUDDY TURNSTONE FORAGING AND AGGRESSIVE BEHAVIOR SARAH GROVES ABSTRACT.--Theforaging behavior of fall migrant Ruddy Turnstoneswas studiedon the Mas- sachusettscoast on 2 different substrates,barnacle-covered rocks and sand and weed-litteredflats. Foragingrates differedsignificantly between the 2 substrates.On eachsubstrate the foragingand successrates of adults and juveniles differed significantly while the frequenciesof successwere similarfor both age-classes.The observeddifferences in foragingrates of adultsand juvenilesmay be due to the degreeof refinementof foragingtechniques. Experience in searchingfor and handling prey may be a primary factor accountingfor thesedifferences, and foragingperformance probably improves with age and experience.Alternatively, the differencesmay be due to the presenceof inefficient juveniles that do not survive to adulthood. Both adultsand juveniles in the tall-depressedposture were dominant in aggressiveinteractions much morefrequently than birds in the tall-levelposture. In mixedflocks of foragingadult and juvenile turnstones,the four possibletypes of aggressiveinteractions occurred nonrandomly. Adult over juvenile interactionsoccurred more frequently than expected,and juvenile over adult interac- tions were never seen.A tentative explanationof this phenomenonmay be that juveniles misinter- pret or respondambivalently to messagesconveyed behaviorally by adultsand thusbecome espe- cially vulnerableto aggressionby adults.The transiencyof migrantsmade it unfeasibleto evaluate -

Fronts in the World Ocean's Large Marine Ecosystems. ICES CM 2007
- 1 - This paper can be freely cited without prior reference to the authors International Council ICES CM 2007/D:21 for the Exploration Theme Session D: Comparative Marine Ecosystem of the Sea (ICES) Structure and Function: Descriptors and Characteristics Fronts in the World Ocean’s Large Marine Ecosystems Igor M. Belkin and Peter C. Cornillon Abstract. Oceanic fronts shape marine ecosystems; therefore front mapping and characterization is one of the most important aspects of physical oceanography. Here we report on the first effort to map and describe all major fronts in the World Ocean’s Large Marine Ecosystems (LMEs). Apart from a geographical review, these fronts are classified according to their origin and physical mechanisms that maintain them. This first-ever zero-order pattern of the LME fronts is based on a unique global frontal data base assembled at the University of Rhode Island. Thermal fronts were automatically derived from 12 years (1985-1996) of twice-daily satellite 9-km resolution global AVHRR SST fields with the Cayula-Cornillon front detection algorithm. These frontal maps serve as guidance in using hydrographic data to explore subsurface thermohaline fronts, whose surface thermal signatures have been mapped from space. Our most recent study of chlorophyll fronts in the Northwest Atlantic from high-resolution 1-km data (Belkin and O’Reilly, 2007) revealed a close spatial association between chlorophyll fronts and SST fronts, suggesting causative links between these two types of fronts. Keywords: Fronts; Large Marine Ecosystems; World Ocean; sea surface temperature. Igor M. Belkin: Graduate School of Oceanography, University of Rhode Island, 215 South Ferry Road, Narragansett, Rhode Island 02882, USA [tel.: +1 401 874 6533, fax: +1 874 6728, email: [email protected]]. -

Population Analysis and Community Workshop for Far Eastern Curlew Conservation Action in Pantai Cemara, Desa Sungai Cemara – Jambi
POPULATION ANALYSIS AND COMMUNITY WORKSHOP FOR FAR EASTERN CURLEW CONSERVATION ACTION IN PANTAI CEMARA, DESA SUNGAI CEMARA – JAMBI Final Report Small Grant Fund of the EAAFP Far Eastern Curlew Task Force Iwan Febrianto, Cipto Dwi Handono & Ragil S. Rihadini Jambi, Indonesia 2019 The aim of this project are to Identify the condition of Far Eastern Curlew Population and the remaining potential sites for Far Eastern Curlew stopover in Sumatera, Indonesia and protect the remaining stopover sites for Far Eastern Curlew by educating the government, local people and community around the sites as the effort of reducing the threat of habitat degradation, habitat loss and human disturbance at stopover area. INTRODUCTION The Far Eastern Curlew (Numenius madagascariencis) is the largest shorebird in the world and is endemic to East Asian – Australian Flyway. It is one of the Endangered migratory shorebird with estimated global population at 38.000 individual, although a more recent update now estimates the population at 32.000 (Wetland International, 2015 in BirdLife International, 2017). An analysis of monitoring data collected from around Australia and New Zealand (Studds et al. in prep. In BirdLife International, 2017) suggests that the species has declined much more rapidly than was previously thought; with an annual rate of decline of 0.058 equating to a loss of 81.7% over three generations. Habitat loss occuring as a result of development is the most significant threat currently affecting migratory shorebird along the EAAF (Melville et al. 2016 in EAAFP 2017). Loss of habitat at critical stopover sites in the Yellow Sea is suspected to be the key threat to this species and given that it is restricted to East Asian - Australasian Flyway, the declines in the non-breeding are to be representative of the global population. -

Common Caribbean Shorebirds: ID Guide
Common Caribbean Shorebirds: ID Guide Large Medium Small 14”-18” 35 - 46 cm 8.5”-12” 22 - 31 cm 6”- 8” 15 - 20 cm Large Shorebirds Medium Shorebirds Small Shorebirds Whimbrel 17.5” 44.5 cm Lesser Yellowlegs 9.5” 24 cm Wilson’s Plover 7.75” 19.5 cm Spotted Sandpiper 7.5” 19 cm American Oystercatcher 17.5” 44.5 cm Black-bellied Plover 11.5” 29 cm Sanderling 7.75” 19.5 cm Western Sandpiper 6.5” 16.5 cm Willet 15” 38 cm Short-billed Dowitcher 11” 28 cm White-rumped Sandpiper 6” 15 cm Greater Yellowlegs 14” 35.5 cm Ruddy Turnstone 9.5” 24 cm Semipalmated Sandpiper 6.25” 16 cm 6.25” 16 cm American Avocet* 18” 46 cm Red Knot 10.5” 26.5 cm Snowy Plover Least Sandpiper 6” 15 cm 14” 35.5 cm 8.5” 21.5 cm Semipalmated Plover Black-necked Stilt* Pectoral Sandpiper 7.25” 18.5 cm Killdeer* 10.5” 26.5 cm Piping Plover 7.25” 18.5 cm Stilt Sandpiper* 8.5” 21.5 cm Lesser Yellowlegs & Ruddy Turnstone: Brad Winn; Red Knot: Anthony Levesque; Pectoral Sandpiper & *not pictured Solitary Sandpiper* 8.5” 21.5 cm White-rumped Sandpiper: Nick Dorian; All other photos: Walker Golder Clues to help identify shorebirds Size & Shape Bill Length & Shape Foraging Behavior Size Length Sandpipers How big is it compared to other birds? Peeps (Semipalmated, Western, Least) Walk or run with the head down, picking and probing Spotted Sandpiper Short Medium As long Longer as head than head Bobs tail up and down when walking Plovers, Turnstone or standing Small Medium Large Sandpipers White-rumped Sandpiper Tail tips up while probing Yellowlegs Overall Body Shape Stilt Sandpiper Whimbrel, Oystercatcher, Probes mud like “oil derrick,” Willet, rear end tips up Dowitcher, Curvature Plovers Stilt, Avocet Run & stop, pick, hiccup, run & stop Elongate Compact Yellowlegs Specific Body Parts Stroll and pick Bill & leg color Straight Upturned Dowitchers Eye size Plovers = larger, sandpipers = smaller Tip slightly Probe mud with “sewing machine” Leg & neck length downcurved Downcurved bill, body stays horizontal . -

Draft Version Target Shorebird Species List
Draft Version Target Shorebird Species List The target species list (species to be surveyed) should not change over the course of the study, therefore determining the target species list is an important project design task. Because waterbirds, including shorebirds, can occur in very high numbers in a census area, it is often not possible to count all species without compromising the quality of the survey data. For the basic shorebird census program (protocol 1), we recommend counting all shorebirds (sub-Order Charadrii), all raptors (hawks, falcons, owls, etc.), Common Ravens, and American Crows. This list of species is available on our field data forms, which can be downloaded from this site, and as a drop-down list on our online data entry form. If a very rare species occurs on a shorebird area survey, the species will need to be submitted with good documentation as a narrative note with the survey data. Project goals that could preclude counting all species include surveys designed to search for color-marked birds or post- breeding season counts of age-classed bird to obtain age ratios for a species. When conducting a census, you should identify as many of the shorebirds as possible to species; sometimes, however, this is not possible. For example, dowitchers often cannot be separated under censuses conditions, and at a distance or under poor lighting, it may not be possible to distinguish some species such as small Calidris sandpipers. We have provided codes for species combinations that commonly are reported on censuses. Combined codes are still species-specific and you should use the code that provides as much information as possible about the potential species combination you designate. -

Characteristics of the Bohai Sea Oil Spill and Its Impact on the Bohai Sea Ecosystem
Article SPECIAL TOPIC: Change of Biodiversity Patterns in Coastal Zone July 2013 Vol.58 No.19: 22762281 doi: 10.1007/s11434-012-5355-0 SPECIAL TOPICS: Characteristics of the Bohai Sea oil spill and its impact on the Bohai Sea ecosystem GUO Jie1,2,3*, LIU Xin1,2,3 & XIE Qiang4,5 1 Key Laboratory of Coastal Zone Environmental Processes, Chinese Academy of Sciences, Yantai 264003, China; 2 Shandong Provincial Key Laboratory of Coastal Zone Environmental Processes, Yantai 264003, China; 3 Yantai Institute of Coastal Zone Research, Chinese Academy of Sciences, Yantai 264003, China; 4 State Key Laboratory of Tropical Oceanography, South China Sea Institute of Oceanology, Chinese Academy of Sciences, Guangzhou 510301, China; 5 Sanya Institute of Deep-sea Science and Engineering, Chinese Academy of Sciences, Sanya 572000, China Received April 26, 2012; accepted June 11, 2012; published online July 16, 2012 In this paper, ENVISAT ASAR data and the Estuary, Coastal and Ocean Model was used to analyze and compare characteristics of the Bohai Sea oil spill. The oil slicks have spread from the point of the oil spill to the east and north-western Bohai Sea. We make a comparison between the changes caused by the oil spill on the chlorophyll concentration and the sea surface temperature using MODIS data, which can be used to analyze the effect of the oil spill on the Bohai Sea ecosystem. We found that the Bohai Sea oil spill caused abnormal chlorophyll concentration distributions and red tide nearby area of oil spill. ENVISAT ASAR, MODIS, oil spill, chlorophyll, sea surface temperature Citation: Guo J, Liu X, Xie Q. -

Growth and Development of Long-Billed Curlew Chicks
April 1973] General Notes 435 Pitelka and Donald L. Beaver critically read the manuscript. This work was con- ducted under the I.B.P. Analysis of Ecosystems-TundraProgram and supported by a grant to F. A. Pitelka from the National ScienceFoundation.--THo•rAs W. CUSTrR, Department o! Zoology and Museum o! Vertebrate Zoology, University o! California, Berkeley,California 94720. Accepted9 May 72. Growth and development of Long-billed Curlew chicks.--Compared with the altricial nestlings of passerinesand the semiprecocialyoung of gulls, few studies of the growth and developmentof the precocialchicks of the Charadrii have been made (Pettingill, 1970: 378). In Europe, yon Frisch (1958, 1959) describedthe develop- ment of behavior in 14 plovers and sandpipers. Davis (1943) and Nice (1962) have reported on the growth of Killdeer (Charadriusvociferus), Nice (1962) on the Spotted Sandpiper (Actiris macularia), and Webster (1942) on the growth and development of plumages in the Black Oystercatcher (Haematopus bachmani). Pettingill (1936) studiedthe atypical AmericanWoodcock (Philohelaminor). Among the curlews, Genus Numenius, only the Eurasian Curlew (N. arquata) has been studied (von Frisch, 1956). Becauseof the scant knowledgeabout the development of the youngin the Charadriiand the scarcityof informationon all aspectsof the breeding biology of the Long-billed Curlew (N. americanus) (Palmer, 1967), I believe that the following data on the growth and development of Long-billed Curlew chicks are relevant. I took four eggs,one being pipped, from a nest 10 miles west of Brigham City, Box Elder County, Utah, on 24 May 1966. One egg was preservedimmediately for additional study, the others I placed in a 4' X 3' X 2' cardboard box with a 60-watt lamp for warmth in a vacant room in my home until they hatched. -

List of Shorebird Profiles
List of Shorebird Profiles Pacific Central Atlantic Species Page Flyway Flyway Flyway American Oystercatcher (Haematopus palliatus) •513 American Avocet (Recurvirostra americana) •••499 Black-bellied Plover (Pluvialis squatarola) •488 Black-necked Stilt (Himantopus mexicanus) •••501 Black Oystercatcher (Haematopus bachmani)•490 Buff-breasted Sandpiper (Tryngites subruficollis) •511 Dowitcher (Limnodromus spp.)•••485 Dunlin (Calidris alpina)•••483 Hudsonian Godwit (Limosa haemestica)••475 Killdeer (Charadrius vociferus)•••492 Long-billed Curlew (Numenius americanus) ••503 Marbled Godwit (Limosa fedoa)••505 Pacific Golden-Plover (Pluvialis fulva) •497 Red Knot (Calidris canutus rufa)••473 Ruddy Turnstone (Arenaria interpres)•••479 Sanderling (Calidris alba)•••477 Snowy Plover (Charadrius alexandrinus)••494 Spotted Sandpiper (Actitis macularia)•••507 Upland Sandpiper (Bartramia longicauda)•509 Western Sandpiper (Calidris mauri) •••481 Wilson’s Phalarope (Phalaropus tricolor) ••515 All illustrations in these profiles are copyrighted © George C. West, and used with permission. To view his work go to http://www.birchwoodstudio.com. S H O R E B I R D S M 472 I Explore the World with Shorebirds! S A T R ER G S RO CHOOLS P Red Knot (Calidris canutus) Description The Red Knot is a chunky, medium sized shorebird that measures about 10 inches from bill to tail. When in its breeding plumage, the edges of its head and the underside of its neck and belly are orangish. The bird’s upper body is streaked a dark brown. It has a brownish gray tail and yellow green legs and feet. In the winter, the Red Knot carries a plain, grayish plumage that has very few distinctive features. Call Its call is a low, two-note whistle that sometimes includes a churring “knot” sound that is what inspired its name. -

Long-Billed Curlew ASSESSING HABITAT QUALITY for PRIORITY WILDLIFE SPECIES in COLORADO WETLANDS
COLORADO PARKS & WILDLIFE Long-billed Curlew ASSESSING HABITAT QUALITY FOR PRIORITY WILDLIFE SPECIES IN COLORADO WETLANDS Species Distribution Range Long-billed curlews breed in the western United States, including eastern Colorado, and in southwestern Canada. During migration, long-billed curlews occur sporadically in western Colorado and regularly throughout eastern Colorado. © “MIKE” MICHAEL L. BAIRD BAIRD L. MICHAEL “MIKE” © Long-billed curlews (Numenius americanus, Family Scolopacidae) have a distinctive long bill that curves downward. They are can be found near playas and ponds in eastern Colorado. insects, particularly grasshoppers. Species Description They also eat some vertebrate species, Identification including fish, amphibians, and bird The long-billed curlew, at 20–26 inches eggs/nestlings. Breeding in length, is the largest shorebird in Winter North America. Their primitive- Conservation Status sounding curlee vocalizations are Populations of long-billed curlews considered a harbinger of spring. Their have experienced overall declines in down-curved, sickle-shaped bill is the many areas, especially throughout the largest among shorebirds and inspired eastern United States, due primarily to their genus name, Numenius, derived habitat loss and historic over-hunting. from the Greek word, noumenios, In Colorado, long-billed curlews are meaning of the new crescent moon. listed as a Tier 2 Species of Great- est Conservation Need (CPW 2015). Preferred Habitats The Breeding Bird Survey indicates a Long-billed curlews are considered a significant population decline in Colo- grassland species, but they are rarely rado, and the Colorado Breeding Bird observed far from water. In Colorado, Atlas indicates a decrease in distribu- they are usually associated with ponds, tion. -

The Following Is a List of Birds Seen Or Reported Around Vinalhaven Over the Past Twenty Years
The following is a list of birds seen or reported around Vinalhaven over the past twenty years. Common Loon (Gavia immer) Snipe (Gallinago gallinago) Red-throated Loon (Gavia stellata) Woodcock (Scolopax minor)N Red Necked Grebe Podiceps grisegena) Ruddy Turnstone (Arenaria interpres) Horned Grebe (Podiceps auritus) Purple Sandpiper (Calidris maritime) Pie- billed Grebe (Poilymbus podiceps)N? Red Knot (Calidris canutus) Yellow-nosed albatross (Diomedi chlororhynchos) Dunlin (Calidris alpina) Fulmar (Fulmarus glacialis) Sanderling (Calidris alba) Sooty Shearwater (Puffinus griseus) Semipalmated Sandpiper (Calidris pusilla) Greater Shearwater (Puffinus gravis) Western Sandpiper (Calidrs mauri) Manx Shearwater (Puffinus puffinus) Least Sandpiper (Calsidris minuttla) Wilson’s Petrel (Oceanites oceanicus) White-rumped Sandpiper (Calidris fuscicollis) Leach’s Petrel (Oceanodroma leucorhoa)N Baird’s Snadpiper (Calidris bairdii) White Pelican (Pelecanus erthrorhynchos) Pectoral Sandpiper (Calidris melnotos) Brown Pelican (Pelecanus occidentalis) Upland Sandpiper (Bartramia longicauda) Gannet (Sula bassanus) Pomarine Jaeger (Stoecorarius pomarinus) Great Cormorant (Phalocrocorax carbo)N Parasitic Jaeger (Stercorarius parasiticus) Double -breasted cormorant (Phalacrocorax auritus)N Little Gull (Larus minutus) Least Bittern (Ixobrychus exilis) Laughing Gull (Larus atricilla)N Bittern (Botaurus lentiginosus) Ring-billed Gull (Larus delawarensis) Black-crowned Night Heron (Nycticorax nycticorax) Herring Gull (Larus argenatatusN Green Heron (Butorides -
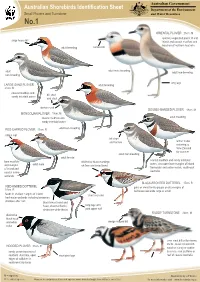
Australian Shorebirds Identification Sheet Department of the Environment Small Plovers and Turnstone and Water Resources No.1
Australian Government Australian Shorebirds Identification Sheet Department of the Environment Small Plovers and Turnstone and Water Resources No.1 ORIENTAL PLOVER 25cm. M sparsely vegetated plains of arid large heavy bill inland and coastal mudflats and beaches of northern Australia adult breeding narrow bill adult male breeding adult adult non-breeding non-breeding long legs LARGE SAND PLOVER adult breeding 21cm. M coastal mudflats and bill short sandy intertidal zones and stout darker mask DOUBLE-BANDED PLOVER 19cm. M MONGOLIAN PLOVER 19cm. M coastal mudflats and adult breeding sandy intertidal zones RED-CAPPED PLOVER 15cm. R adult non-breeding rufous cap bill short and narrow winter visitor returning to New Zealand for summer adult non-breeding adult female coastal mudflats and sandy intertidal bare mudflats distinctive black markings zones, also open bare margins of inland and margins adult male on face and breastband of inland and freshwater and saline marsh, south-east coastal saline Australia wetlands BLACK-FRONTED DOTTEREL 17cm. R RED-KNEED DOTTEREL pairs or small family groups on dry margins of 18cm. R feshwater wetlands large or small feeds in shallow margins of inland short rear end freshwater wetlands including temporary shallows after rain black breast band and head, chestnut flanks, long legs with distinctive white throat pink upper half RUDDY TURNSTONE 23cm. M distinctive black hood and white wedge shaped bill collar uses stout bill to flip stones, shells, seaweed and drift- 21cm. R HOODED PLOVER wood on sandy or cobble sandy ocean beaches of beaches, rock platform or southern Australia, open short pink legs reef of coastal Australia edges of saltlakes in south-west Australia M = migratory .