Johnson & Johnson
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Missing Pieces Report: the 2018 Board Diversity Census of Women
alliance for board diversity Missing Pieces Report: The 2018 Board Diversity Census of Women and Minorities on Fortune 500 Boards Missing Pieces Report: The 2018 Board Diversity Census of Women and Minorities on Fortune 500 Boards About the Alliance for Board Diversity Founded in 2004, the Alliance for Board Diversity (ABD) is a collaboration of four leadership organizations: Catalyst, The Executive Leadership Council (ELC), the Hispanic Association on Corporate Responsibility (HACR), and LEAP (Leadership Education for Asian Pacifics). Diversified Search, an executive search firm, is a founding partner of the alliance and serves as an advisor and facilitator. The ABD’s mission is to increase the representation of women and minorities on corporate boards. More information about ABD is available at www.theabd.org. About Deloitte In the US, Deloitte LLP and Deloitte USA LLP are member firms of DTTL. The subsidiaries of Deloitte LLP provide industry- leading audit & assurance, consulting, tax, and risk and financial advisory services to many of the world’s most admired brands, including more than 85 percent of the Fortune 500 and more than 6,000 private and middle market companies. Our people work across more than 20 industry sectors with one purpose: to deliver measurable, lasting results. We help reinforce public trust in our capital markets, inspire clients to make their most challenging business decisions with confidence, and help lead the way toward a stronger economy and a healthy society. As part of the DTTL network of member firms, we are proud to be associated with the largest global professional services network, serving our clients in the markets that are most important to them. -

From the Academy
FROM THE ACADEMY Joint American Academy of DermatologyeNational Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy Craig A. Elmets, MD (Co-Chair),a HenryW.Lim,MD,b Benjamin Stoff, MD, MA,c Cody Connor, MD,a Kelly M. Cordoro, MD,d Mark Lebwohl, MD,e AprilW.Armstrong,MD,MPH,f Dawn M. R. Davis, MD,g Boni E. Elewski, MD,a Joel M. Gelfand, MD, MSCE,h Kenneth B. Gordon, MD,i AliceB.Gottlieb,MD,PhD,j Daniel H. Kaplan, MD, PhD,k Arthur Kavanaugh, MD,l Matthew Kiselica, BA/BS,m Dario Kivelevitch, MD,n Neil J. Korman, MD, PhD,o Daniela Kroshinsky, MD, MPH,p Craig L. Leonardi, MD,q Jason Lichten, MD,m NehalN.Mehta,MD,MSCE,r Amy S. Paller, MD,s Sylvia L. Parra, MD,t Arun L. Pathy, MD,u Elizabeth A. Farley Prater, MD,v Reena N. Rupani, MD,e Michael Siegel, PhD,m BruceE.Strober,MD,PhD,w,x Emily B. Wong, MD,y Jashin J. Wu, MD,z Vidhya Hariharan, PhD,aa and Alan Menter, MD (Co-Chair)n Birmingham, Alabama; Detroit, Michigan; Atlanta, Georgia; San Francisco, Los Angeles, San Diego, and Irvine, California; New York, New York; Rochester, Minnesota; Philadelphia and Pittsburgh, Pennsylva- nia; Milwaukee, Wisconsin; Portland, Oregon; Dallas and San Antonio, Texas; Cleveland, Ohio; Boston, Massachusetts; St. Louis, Missouri; Bethesda, Maryland; Chicago and Rosemont, Illinois; Sumter, South Carolina; Centennial, Colorado; Oklahoma City, Oklahoma; Farmington, Connecticut; and Waterloo, Ontario, Canada Psoriasis is a chronic inflammatory disease involving multiple organ systems and affecting approximately 3.2% of the world’s population. -

Mouth Rinses
Mouth Rinses We may recommend a mouth rinse to you for one or more of the following reasons: Periodontal Disease, Gingivitis, Halitosis (bad breath), sensitive teeth, decay or increased risk of decay, dry mouth, minor mouth sore or irritation. Red= RX only Blue= Available through your dentist only Green= Over the counter *Common side effects found with many mouth rinses include the possibility of staining. We still encourage you to use the rinse that treats your problem because none of the stains these rinses cause are permanent. With good oral hygiene on your part, using your bleaching trays and bleach as needed, you can keep the staining to a minimum. If there is any staining that you can’t prevent, it can always be easily removed by your hygienist at your next cleaning. If you have periodontal disease or Gingivitis, we may recommend an antimicrobial/antiseptic type rinse. These rinses kill germs, reduce plaque, redness, swelling and bleeding when used in conjunction with proper brushing and flossing. Some common examples of antiseptic rinses are: Peridex or Perioguard oral rinses are available by prescription only. Active ingredient is chlorhexidine gluconate .12%. These also contain alcohol 11.6%. Can cause staining of the teeth*, taste alteration and dry mouth. Periomed Oral rinse is available through your dentist only. Active ingredient is stannous fluoride .63%. It is alcohol free. Periomed provides antimicrobial activity for up to 8 hours. Also promotes enamel remineralization, and helps reduce sensitivity. Can cause staining* and dry mouth. Listerine Antiseptic: Active ingredient is alcohol 26.9% in the original gold Listerine, and 21.6% in the other flavors. -

2015 Annual Report
ANNUAL REPORT 2015 MARCH 2016 TO OUR SHAREHOLDERS ALEX GORSKY Chairman, Board of Directors and Chief Executive Officer This year at Johnson & Johnson, we are proud this aligned with our values. Our Board of WRITTEN OVER to celebrate 130 years of helping people Directors engages in a formal review of 70 YEARS AGO, everywhere live longer, healthier and happier our strategic plans, and provides regular OUR CREDO lives. As I reflect on our heritage and consider guidance to ensure our strategy will continue UNITES & our future, I am optimistic and confident in the creating better outcomes for the patients INSPIRES THE long-term potential for our business. and customers we serve, while also creating EMPLOYEES long-term value for our shareholders. OF JOHNSON We manage our business using a strategic & JOHNSON. framework that begins with Our Credo. Written OUR STRATEGIES ARE BASED ON over 70 years ago, it unites and inspires the OUR BROAD AND DEEP KNOWLEDGE employees of Johnson & Johnson. It reminds OF THE HEALTH CARE LANDSCAPE us that our first responsibility is to the patients, IN WHICH WE OPERATE. customers and health care professionals who For 130 years, our company has been use our products, and it compels us to deliver driving breakthrough innovation in health on our responsibilities to our employees, care – from revolutionizing wound care in communities and shareholders. the 1880s to developing cures, vaccines and treatments for some of today’s most Our strategic framework positions us well pressing diseases in the world. We are acutely to continue our leadership in the markets in aware of the need to evaluate our business which we compete through a set of strategic against the changing health care environment principles: we are broadly based in human and to challenge ourselves based on the health care, our focus is on managing for the results we deliver. -

Johnson & Johnson
JOHNSON & JOHNSON FORM 10-K (Annual Report) Filed 02/22/13 for the Period Ending 12/30/12 Address ONE JOHNSON & JOHNSON PLZ NEW BRUNSWICK, NJ 08933 Telephone 732-524-2455 CIK 0000200406 Symbol JNJ SIC Code 2834 - Pharmaceutical Preparations Industry Biotechnology & Drugs Sector Healthcare Fiscal Year 12/12 http://www.edgar-online.com © Copyright 2013, EDGAR Online, Inc. All Rights Reserved. Distribution and use of this document restricted under EDGAR Online, Inc. Terms of Use. UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 10-K ANNUAL REPORT PURSUANT TO SECTION 13 OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended December 30, 2012 Commission file number 1-3215 JOHNSON & JOHNSON (Exact name of registrant as specified in its charter) New Jersey 22-1024240 (State of incorporation) (I.R.S. Employer Identification No.) One Johnson & Johnson Plaza New Brunswick, New Jersey 08933 (Address of principal executive offices) (Zip Code) Registrant’s telephone number, including area code: (732) 524-0400 SECURITIES REGISTERED PURSUANT TO SECTION 12(b) OF THE ACT Title of each class Name of each exchange on which registered Common Stock, Par Value $1.00 New York Stock Exchange Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes No Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes No Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Exchange Act during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. -

Annual Report on Annual Reports 2016
ANNUAL REPORT ON ANNUAL REPORTS 2016 TOP 400 ANNUAL REPORTS WHO RANKS WHERE? 100 ANNUALS IN BRIEF BEST REPORTING PRACTICES Company Value > Report Value Annual Report on Annual Reports 2016 Contents Report rating scale 3 Top 400 annual reports 4 Who ranks where? 25 From ABB to ZTE 100 annuals in brief 58 From Abbott to Yamaha How important is the annual report today? 92 Views from Cecilia Ketels, Kellie Friery, Renee Carter, David Robinson, Kaevan Gazdar, Elena Moskvina, Thomas Rosenmayr, Rob Stangroom, Andrey Kozhevnikov, Ana Santamarina, Katie Holcomb, Ananda Jagoda Best practices on key report attibutes 100 Strategy, message, investor information, risks, style, online… How we make it 121 How is your report doing? The report scan 127 The report rating panel 128 Robert Berick, Susan Blesener, Renee Carter, Vero Escarmelle, Helena Fournial, Kaevan Gazdar, Mike Guillaume, Pradip Seth, Eva Wolosiuk Making reports pay off 133 e.com – ReportWatch 135 2 Report rating scale A+ ééééé First-rate A éééé(é) Excellent A- éééé Very good B+ ééé(é) Sound B ééé Average B- éé(é) Uneven C+ éé Common C é(é) Substandard C- é Poor D (é) Uncompetitive 3 Top 400 annual reports AkzoNobel (No. 1) Electrolux (No. 2 ) SCA (No. 3) Volvo (No. 4) 4 Report rank Company Country Report rating Compare 1 AKZONOBEL Netherlands A+ DUPONT 2 ELECTROLUX Sweden A+ WHIRLPOOL 3 SCA Sweden A+ KIMBERLY-CLARK 4 VOLVO Sweden A+ DAIMLER 5 POTASHCORP Canada A+ AGRIUM 6 ATLAS COPCO Sweden A SANDVIK 7 STORA ENSO Finland A UPM 8 BOLIDEN Sweden A GLENCORE 9 WIENERBERGER Austria A BORAL 10 -

Missing Pieces Report: the Board Diversity Census of Women And
Missing Pieces Report: The Board Diversity Census of Women and Minorities on Fortune 500 Boards, 6th edition Missing Pieces Report: The Board Diversity Census of Women and Minorities on Fortune 500 Boards, 6th edition About the Alliance for Board Diversity Founded in 2004, the Alliance for Board Diversity (ABD) is a collaboration of four leadership organizations: Catalyst, the Executive Leadership Council (ELC), the Hispanic Association on Corporate Responsibility (HACR), and Leadership Education for Asian Pacifics (LEAP). Diversified Search Group, an executive search firm, is a founding partner of the alliance and serves as an advisor and facilitator. The ABD’s mission is to enhance shareholder value in Fortune 500 companies by promoting inclusion of women and minorities on corporate boards. More information about ABD is available at theabd.org. About Deloitte Deloitte provides industry-leading audit, consulting, tax and advisory services to many of the world’s most admired brands, including nearly 90% of the Fortune 500® and more than 7,000 private companies. Our people come together for the greater good and work across the industry sectors that drive and shape today’s marketplace — delivering measurable and lasting results that help reinforce public trust in our capital markets, inspire clients to see challenges as opportunities to transform and thrive, and help lead the way toward a stronger economy and a healthier society. Deloitte is proud to be part of the largest global professional services network serving our clients in the markets that are most important to them. Now celebrating 175 years of service, our network of member firms spans more than 150 countries and territories. -

1 Brief Report: the Virucidal Efficacy of Oral Rinse Components Against SARS-Cov-2 in Vitro Evelina Statkute1†, Anzelika Rubin
bioRxiv preprint doi: https://doi.org/10.1101/2020.11.13.381079; this version posted November 13, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. Brief Report: The Virucidal Efficacy of Oral Rinse Components Against SARS-CoV-2 In Vitro Evelina Statkute1†, Anzelika Rubina1†, Valerie B O’Donnell1, David W. Thomas2† Richard J. Stanton1† 1Systems Immunity University Research Institute, Division of Infection & Immunity, School of Medicine, Heath Park, Cardiff, CF14 4XN 2Advanced Therapies Group, School of Dentistry, Cardiff University, Heath Park, Cardiff CF14 4XY, UK †These authors contributed equally * Correspondence: [email protected], [email protected] Running title: Virucidal Activity of Mouthwashes Keywords: SARS-CoV2, mouthwash, lipid, envelope Disclosure: Venture Life Group plc provided information on mouthwash formulations employed in the study, but had no role in funding, planning, execution, analysis or writing of this study. A separate study funded to Cardiff University by Venture Life Group is assessing in vivo efficacy of CPC in patients with COVID19. The investigators declare no direct conflicts exist. 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.11.13.381079; this version posted November 13, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-ND 4.0 International license. -

Notes on Numbers 202 1 Edition Dr
Notes on Numbers 202 1 Edition Dr. Thomas L. Constable TITLE The title the Jews used in their Hebrew Old Testament for this book comes from the fifth word in the book in the Hebrew text, bemidbar: "in the wilderness." This is, of course, appropriate since the Israelites spent most of the time covered in the narrative of Numbers in the wilderness. The English title "Numbers" is a translation of the Greek title Arithmoi. The Septuagint translators chose this title because of the two censuses of the Israelites that Moses recorded at the beginning (chs. 1—4) and toward the end (ch. 26) of the book. These "numberings" of the people took place at the beginning and end of the wilderness wanderings and frame the contents of Numbers. DATE AND WRITER Moses wrote Numbers (cf. Num. 1:1; 33:2; Matt. 8:4; 19:7; Luke 24:44; John 1:45; et al.). He apparently wrote it late in his life, across the Jordan from the Promised Land, on the Plains of Moab.1 Moses evidently died close to 1406 B.C., since the Exodus happened about 1446 B.C. (1 Kings 6:1), the Israelites were in the wilderness for 40 years (Num. 32:13), and he died shortly before they entered the Promised Land (Deut. 34:5). There are also a few passages that appear to have been added after Moses' time: 12:3; 21:14-15; and 32:34-42. However, it is impossible to say how much later. 1See the commentaries for fuller discussions of these subjects, e.g., Gordon J. -
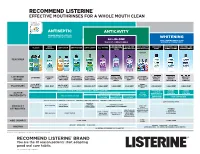
Listerine Floss Product Chart EN V9black
RECOMMEND LISTERINE® EFFECTIVE MOUTHRINSES FOR A WHOLE MOUTH CLEAN® 3 ANTISEPTIC ANTICAVITY 2X MORE HEALTHY SITES VS. JUST BRUSHING & FLOSSING WHITENING ALL-IN-ONE ONLY LEADING BRAND THAT THE MOST COMPLETE RINSE WHITENS & STRENGTHENS ALL-IN-ONE ZERO ALL-IN-ONE ANTI-CAVITY PEROXIDE WHITENS AND WHITENS AND CLASSIC ANTI-STAIN ANTI-TARTAR ANTI-CAVITY ALL-IN-ONE FOR SENSITIVE ALCOHOL TEETH ZERO ALCOHOL ZERO ALCOHOL FREE STRENGTHENS STRENGTHENS METERED DOSING FOR KIDS FEATURES ® LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE LISTERINE TOTAL CARE HEALTHY HEALTHY HEALTHY ULTRACLEAN ULTRACLEAN ULTRACLEAN TOTAL CARE SMART RINSE ZERO TOTAL CARE FOR SENSITIVE WHITE™ WHITE™ WHITE™ BRAND ANTI-STAIN ANTI-TARTAR ANTI-CAVITY TEETH ZERO FOR KIDS GENTLE RESTORING VIBRANT COOL MINT BERRY FRESHBURST ARCTIC MINT BUBBLE GUM FLAVOURS MILD MINT COOL MINT FRESHBURST CLEAN MINT CLEAN MINT MILD MINT CLEAN MINT CLEAN MINT CLEAN MINT ORIGINAL COOL CITRUS MINT EUCALYPTOL 0.091% W/V MENTHOL 0.042% W/V THYMOL 0.063% W/V SODIUM FLUORIDE SODIUM SODIUM 0.02% W/W FLUORIDE FLUORIDE ACTIVE SODIUM FLUORIDE 0.022% W/V SODIUM TETRAPOTASSIUM 0.022% W/V 0.02% W/W FLUORIDE PYROPHOSPHATE INGREDIENTS ZINC CHLORIDE 0.09% W/V ZINC CHLORIDE POTASSIUM ZINC CHLORIDE 0.022% W/V PENTASODIUM HYDROGEN PEROXIDE 0.09% W/V NITRATE 2.4% W/V 0.09% W/V TRIPHOSPHATE KILLS UP TO 99.9% OF GERMS IN YOUR MOUTH PREVENTS & REDUCES GINGIVITIS PREVENTS & REDUCES PLAQUE SAFELY WHITENS TEETH† PREVENTS CAVITIES MILD FLAVOUR HELPS KEEP PREVENTS CAVITIES PRODUCT TEETH WHITE STRENGTHENS STRENGTHENS TOOTH ENAMEL* 2X BETTER TOOTH ENAMEL CAVITY ATTRIBUTES PROTECTION VS. -

SEC Form 10-K Annual Report
e10vk 10-K 1 y80744e10vk.htm FORM 10-K Table of Contents UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 10-K ANNUAL REPORT PURSUANT TO SECTION 13 OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended January 3, 2010 Commission file number 1-3215 JOHNSON & JOHNSON (Exact name of registrant as specified in its charter) New Jersey 22-1024240 (State of incorporation) (I.R.S. Employer Identification No.) One Johnson & Johnson Plaza New Brunswick, New Jersey 08933 (Address of principal executive offices) (Zip Code) Registrant’s telephone number, including area code: (732) 524-0400 SECURITIES REGISTERED PURSUANT TO SECTION 12(b) OF THE ACT Title of each class Name of each exchange on which registered Common Stock, Par Value $1.00 New York Stock Exchange Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes þ No o Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes o No þ Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Exchange Act during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes þ No o Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). -
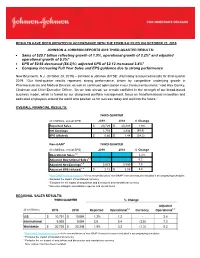
Sales of $20.7 Billion Reflecting Growth of 1.9%, Operational Growth of 3.2
RESULTS HAVE BEEN UPDATED IN ACCORDANCE WITH THE FORM 8-K FILED ON OCTOBER 23, 2019 JOHNSON & JOHNSON REPORTS 2019 THIRD-QUARTER RESULTS: • Sales of $20.7 billion reflecting growth of 1.9%, operational growth of 3.2%* and adjusted operational growth of 5.2%* • EPS of $0.66 decreased (54.2)%; adjusted EPS of $2.12 increased 3.4%* • Company increasing Full Year Sales and EPS guidance due to strong performance New Brunswick, N.J. (October 23, 2019) – Johnson & Johnson (NYSE: JNJ) today announced results for third-quarter 2019. “Our third-quarter results represent strong performance, driven by competitive underlying growth in Pharmaceuticals and Medical Devices, as well as continued optimization in our Consumer business,” said Alex Gorsky, Chairman and Chief Executive Officer. “As we look ahead, we remain confident in the strength of our broad-based business model, which is fueled by our disciplined portfolio management, focus on transformational innovation and dedicated employees around the world who position us for success today and well into the future.” OVERALL FINANCIAL RESULTS: THIRD QUARTER ($ in Millions, except EPS) 2019 2018 % Change Reported Sales $ 20,729 $ 20,348 1.9% Net Earnings 1,753 3,934 (55.4) EPS (diluted) $ 0.66 $ 1.44 (54.2) Non-GAAP* THIRD QUARTER ($ in Millions, except EPS) 2019 2018 % Change Operational Sales1,2 3.2% Adjusted Operational Sales1,3 5.2 Adjusted Net Earnings1,4 5,672 5,590 1.5 Adjusted EPS (diluted)1,4 $ 2.12 $ 2.05 3.4 1 Non-GAAP financial measure; refer to reconciliations of non-GAAP financial measures