A Novel Oxidizing Reagent Based on Potassium Ferrate(VI)1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Decolorization of Reactive Orange 16 Via Ferrate(VI) Oxidation Assisted by Sonication
Turkish Journal of Chemistry Turk J Chem (2017) 41: 577 { 586 http://journals.tubitak.gov.tr/chem/ ⃝c TUB¨ ITAK_ Research Article doi:10.3906/kim-1701-8 Decolorization of reactive orange 16 via ferrate(VI) oxidation assisted by sonication Serkan S¸AHINKAYA_ ∗ Department of Environmental Engineering, Faculty of Engineering and Architecture, Nev¸sehirHacı Bekta¸sVeli University, Nev¸sehir,Turkey Received: 02.01.2017 • Accepted/Published Online: 24.03.2017 • Final Version: 05.09.2017 Abstract: The decolorization of azo dye C.I. reactive orange 16 (RO 16) via ferrate(VI) and sono-ferrate(VI) methods, which is the combination of the ferrate(VI) oxidation method with sonication, has been achieved in the present study. The influences of some important operating parameters, which are the initial pH, the concentration of potassium ferrate(VI) (K 2 FeO 4) and the RO 16 dye, and ultrasonic density (for only the sono-ferrate(VI) method), on the color removal have −1 been investigated. The optimum conditions have been determined as pH = 7 and [K 2 FeO 4 ] = 50 mg L for the −1 −1 individual ferrate(VI) oxidation method and pH = 7 and [K 2 FeO 4 ] = 50 mg L by direct sonication at 0.50 W mL ultrasonic density and 20 kHz fixed frequency for the sono-ferrate(VI) method. The color removal efficiencies were 85% by ferrate(VI) method and 91% by sono-ferrate(VI) method. Kinetic studies were also performed for the decolorization of RO 16 under the optimized conditions at room temperature. It was seen that the oxidative decolorization of RO 16 via the sono-ferrate(VI) method happened more rapidly because of the production of OH • radical through sonication compared to the individual ferrate(VI) method. -

Kinetic Studies on Permanganate Oxidation of Acetophenones Under
Indi an Journal of Chemistry Vol. 40A, June 2001, pp. 610-612 Kinetic studies on permanganate oxidation of The ketones were further purified by vacuum acetophenones under phase transfer catalysis distillation. The solvents employed were purified by standard methods and doubly distilled water was P S Sheeba & T D Radhakrishnan Nair* always used. The catalysts used were tricapryl Department of Chemistry, Calicut University methylammonium chloride (TCMAC) and tetrabutyl Calicut University P.O., Kerala 673 635. India ammonium bromide (TBAB). The former has Received 18 October 2000; revised 8 March 2001 relatively larger organic structure (C 10H21 )JN+CH 3Cr compared to the latter (C4 H9) 4N+B(. Kinetic studies on the permanganate oxidation of The solutions containing the permanganate ions in acetopheno_ne and some of its substituents in organic media using the organic solvents were prepared by shaking the techn1que of phase transfer catalysis are reported. aqueous potassium permanganate solution with the Tncaprylmethylammonium chloride and tetrabutylammonium bromide have been used as the phase transfer catalysts. The organic solvents contammg the phase transfer 4 reaction shows first order dependence each on [ketones] and the catalysts as reported elsewhere . The solutions of the [permanganate ions] respectively . The rate coefficients fit well oxidant thus prepared in the organic solvent and the with the Hammett equation and the p-value calculated aorees well substrate in the organic solvent were thermally with the reaction requirements. "' equilibrated (± 0.05°C) at the desired temperature for Potassium permanganate is a powerful oxidizing about half an hour before mixing. Kinetic runs were carried out under pseudo-first order conditions. -

Removal of 2, 4-Dinitrophenol by Ferrate
University of Central Florida STARS Electronic Theses and Dissertations, 2004-2019 2008 Removal Of 2, 4-dinitrophenol By Ferrate Gianna Cooley University of Central Florida Part of the Environmental Engineering Commons Find similar works at: https://stars.library.ucf.edu/etd University of Central Florida Libraries http://library.ucf.edu This Masters Thesis (Open Access) is brought to you for free and open access by STARS. It has been accepted for inclusion in Electronic Theses and Dissertations, 2004-2019 by an authorized administrator of STARS. For more information, please contact [email protected]. STARS Citation Cooley, Gianna, "Removal Of 2, 4-dinitrophenol By Ferrate" (2008). Electronic Theses and Dissertations, 2004-2019. 3619. https://stars.library.ucf.edu/etd/3619 REMOVAL OF 2, 4-DINITROPHENOL BY FERRATE by GIANNA GRIFFITH COOLEY B.S. Loyola Univeristy New Orleans, 2002 A thesis submitted in partial fulfillment of the requirements for the degree of Master of Environmental Engineering in the Department of Civil and Environmental Engineering in the College of Engineering and Computer Science at the University of Central Florida Orlando, Florida Fall Term 2008 Major Professor: Debra R. Reinhart © 2008 Gianna Griffith Cooley ii ABSTRACT VI 2- Ferrate (molecular formula, Fe O4 ) has been studied increasingly since the 1970s as a disinfectant and coagulant for domestic wastewater and also as an oxidant for industrial wastewaters (Murmann and Roginson, 1974, Gilbert et al., 1978, Kazama, 1994, Jiang et al., 2002, and Sharmaet al., 2005). This research was performed to explore whether ferrate could possibly be used as chemical treatment for industrial wastewaters from plastic, chemical, dye, soap, and wood stain producing plants that contain 2, 4-Dinitrophenol (DNP). -

Gasket Chemical Services Guide
Gasket Chemical Services Guide Revision: GSG-100 6490 Rev.(AA) • The information contained herein is general in nature and recommendations are valid only for Victaulic compounds. • Gasket compatibility is dependent upon a number of factors. Suitability for a particular application must be determined by a competent individual familiar with system-specific conditions. • Victaulic offers no warranties, expressed or implied, of a product in any application. Contact your Victaulic sales representative to ensure the best gasket is selected for a particular service. Failure to follow these instructions could cause system failure, resulting in serious personal injury and property damage. Rating Code Key 1 Most Applications 2 Limited Applications 3 Restricted Applications (Nitrile) (EPDM) Grade E (Silicone) GRADE L GRADE T GRADE A GRADE V GRADE O GRADE M (Neoprene) GRADE M2 --- Insufficient Data (White Nitrile) GRADE CHP-2 (Epichlorohydrin) (Fluoroelastomer) (Fluoroelastomer) (Halogenated Butyl) (Hydrogenated Nitrile) Chemical GRADE ST / H Abietic Acid --- --- --- --- --- --- --- --- --- --- Acetaldehyde 2 3 3 3 3 --- --- 2 --- 3 Acetamide 1 1 1 1 2 --- --- 2 --- 3 Acetanilide 1 3 3 3 1 --- --- 2 --- 3 Acetic Acid, 30% 1 2 2 2 1 --- 2 1 2 3 Acetic Acid, 5% 1 2 2 2 1 --- 2 1 1 3 Acetic Acid, Glacial 1 3 3 3 3 --- 3 2 3 3 Acetic Acid, Hot, High Pressure 3 3 3 3 3 --- 3 3 3 3 Acetic Anhydride 2 3 3 3 2 --- 3 3 --- 3 Acetoacetic Acid 1 3 3 3 1 --- --- 2 --- 3 Acetone 1 3 3 3 3 --- 3 3 3 3 Acetone Cyanohydrin 1 3 3 3 1 --- --- 2 --- 3 Acetonitrile 1 3 3 3 1 --- --- --- --- 3 Acetophenetidine 3 2 2 2 3 --- --- --- --- 1 Acetophenone 1 3 3 3 3 --- 3 3 --- 3 Acetotoluidide 3 2 2 2 3 --- --- --- --- 1 Acetyl Acetone 1 3 3 3 3 --- 3 3 --- 3 The data and recommendations presented are based upon the best information available resulting from a combination of Victaulic's field experience, laboratory testing and recommendations supplied by prime producers of basic copolymer materials. -

Manufacturing of Potassium Permanganate Kmno4 This Is the Most Important and Well Known Salt of Permanganic Acid
Manufacturing of Potassium Permanganate KMnO4 This is the most important and well known salt of permanganic acid. It is prepared from the pyrolusite ore. It is prepared by fusing pyrolusite ore either with KOH or K2CO3 in presence of atmospheric oxygen or any other oxidising agent such as KNO3. The mass turns green with the formation of potassium manganate, K2MnO4. 2MnO2 + 4KOH + O2 →2K2MnO4 + 2H2O 2MnO2 + 2K2CO3 + O2 →2K2MnO4 + 2CO2 The fused mass is extracted with water. The solution is now treated with a current of chlorine or ozone or carbon dioxide to convert manganate into permanganate. 2K2MnO4 + Cl2 → 2KMnO4 + 2KCl 2K2MnO4 + H2O + O3 → 2KMnO4 + 2KOH + O2 3K2MnO4 + 2CO2 → 2KMnO4 + MnO2 + 2K2CO3 Now-a-days, the conversion is done electrolytically. It is electrolysed between iron cathode and nickel anode. Dilute alkali solution is taken in the cathodic compartment and potassium manganate solution is taken in the anodic compartment. Both the compartments are separated by a diaphragm. On passing current, the oxygen evolved at anode oxidises manganate into permanganate. At anode: 2K2MnO4 + H2O + O → 2KMnO4 + 2KOH 2- - - MnO4 → MnO4 + e + - At cathode: 2H + 2e → H2 Properties: It is purple coloured crystalline compound. It is fairly soluble in water. When heated alone or with an alkali, it decomposes evolving oxygen. 2KMnO4 → K2MnO4 + MnO2 + O2 4KMnO4 + 4KOH → 4K2MnO4 + 2H2O + O2 On treatment with conc. H2SO4, it forms manganese heptoxide via permanganyl sulphate which decomposes explosively on heating. 2KMnO4+3H2SO4 → 2KHSO4 + (MnO3)2SO4 + 2H2O (MnO3)2SO4 + H2O → Mn2O7 + H2SO4 Mn2O7 → 2MnO2 + 3/2O2 Potassium permanganate is a powerful oxidising agent. A mixture of sulphur, charcoal and KMnO4 forms an explosive powder. -

(12) Patent Application Publication (10) Pub. No.: US 2011/005250.6 A1 Abel Et Al
US 2011 0052506A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2011/005250.6 A1 Abel et al. (43) Pub. Date: Mar. 3, 2011 (54) STABLE AEROSOL TOPICAL FOAMS Publication Classification COMPRISINGA HYPOCHLORITE SALT (51) Int. Cl. (75) Inventors: Douglas Abel, Sudbury, MA (US); st 94;O CR Ronald M. Gurge, Franklin, MA ( .01) (US); Mark W. Trumbore, A6IP3L/00 (2006.01) Westford, MA (US) (52) U.S. Cl. .......................................................... 424/45 (73) Assignee: Collegium Pharmaceutical, Inc., (57) ABSTRACT Cumberland, RI (US) Described herein are compositions useful in the treatment of atopic dermatitis and other skin conditions, which composi (21) Appl. No.: 12/872,566 tions exhibit enhanced stability. The compositions contain a 1-1. hypochlorite salt, useful for its antimicrobial properties, and (22) Filed: Aug. 31, 2010 are non-irritating when applied to the skin. The compositions O O also provide enhanced moisturizing properties. The compo Related U.S. Application Data sitions can be formulated into a topical aerosol foam with (60) Provisional application No. 61/238,439, filed on Aug. inert, non-flammable propellants, such as hydrofluoroal 31, 2009. kanes, and may be used in cosmetics or pharmaceuticals. US 2011/005250.6 A1 Mar. 3, 2011 STABLE AEROSOL TOPCAL FOAMS itch associated with atopic dermatitis; however, they can COMPRISINGA HYPOCHLORITE SALT cause sleepiness and may not help in all cases of atopic dermatitis. RELATED APPLICATION 0007 For mild cases of atopic dermatitis, an over-the counterformulation of coal taris often used. Coaltar has long 0001. This application claims benefit under 35 U.S.C. 119 been a treatment for a variety of skin conditions. -

Experiment: Determination of Manganese in Steel
p.1 of 4 Chemistry 201 – Winter 2007 Experiment: Determination of Manganese in Steel Manganese (Mn) in steel may be determined upon dissolution as manganese (VII) after oxidation from the manganese oxidation state (II). This procedure calls for three oxidizing agents. Manganese (VII) may be determined spectrophotometrically. During dissolution of the sample with dilute nitric acid to form iron (II) and manganese (II), nitrogen oxide gases are formed. These must be removed, partially by boiling, and the rest with ammonium peroxydisulfate which also removes carbon or other organic matter present. The nitrogen oxides may react with periodic acid which is used to convert manganese to the (VII) oxidation state. Excess peroxydisulfate is decomposed by boiling. The equations are: - + 2+ 3 Mn + 2 NO3 + 8H ---> 3 Mn + 2 NO (g) + 4H2O 2- - 2- + 2 NO2 (g) + S2O8 + 2 H2O ---> 2 NO3 + 2 SO4 + 4 H 2- 2- + 2 S2O8 + 2 H2O + heat ---> 4 SO4 + O2 + 4 H 2+ - - + - 2 Mn + 5 IO4 + 3 H2O ---> 2 MnO4 + 6 H + 5 IO3 NO(colorless gas) + 1/2 O2 (g) <---> NO2 (brown gas) Note: Peroxydisulfate has the required oxidizing potential to change the oxidation state of manganese from (II) to (IV), but the reaction is too slow. Silver could be employed catalytically but the results are too erratic. Periodate oxidizes the manganese to the (VII) state quantitatively and rapidly at the boiling point, and it is advisable to add the sparingly soluble periodate salt in several portions to maintain an excess and to allow for some decomposition of the latter salt at the elevated temperatures. -

Rubber and Composite Hose Chemical Resistant Chart
Rubber and Composite Hose Chemical Resistant Chart Cedar Rapids, IA Corporate Headquarters Phone: 319.365.0471 Toll Free: 800.553.5455 Fax: 319.365.2522 Website: www.apache-inc.com 99004032 rev032713 Chemical Resistance Information This Apache document provides essential information that will facilitate the safe use of rubber and composite type chemical hoses. Chemical hose users are cautioned that this Chemical Resistant has be developed from generallty accepted industry standards. The ratings listed beneath each Elastomer are the base ratings for the chemical listed. This rating is based on the application temperature not exceeding 70ºF (21.1ºC) unless otherwise specified. The degree an Elastomer will resist the effects of a of a specific chemical depends on several variables.It is recommended that a hose with the highest resistant tube to the chemical transferred be used in the application for safety. 1. Concentration of the chemical is very significant (some chemicals may react with an Elastomer differently based on the level of concentration). 2. Temperature - as the temperature increases the deteriorative effect of a chemical may greatly increase on an Elastomer. 3. Time - the longer the duration the chemical is in contact with the Elastomer, the greater the deteriorative effect. 4. Stability of the Chemical - Chemical solutions (combining of different chemicals) may increase the deteriorative effect. 5. Elastomer Grade - There are different grades of specific Elastomer used in hose. The grade of Elastomer used may effect the resistance level of the hose to a specific chemical. It is recommended that only hose listed for chemical service be use. 6. Safety a. -

Physical Sciences Applications 09:00 - 12:00 Thursday, 26Th November, 2020 Track Physical Sciences Applications Presentation Type Oral Presentation
Physical Sciences Applications 09:00 - 12:00 Thursday, 26th November, 2020 Track Physical Sciences Applications Presentation type Oral Presentation 09:00 - 09:15 192 Quantifying ordering phenomena in lanthanum barium ferrate at the atomic scale Judith Lammer1, Christian Berger2, Stefan Löffler3, Daniel Knez1, Paolo Longo4, Edith Bucher2, Gerald Kothleitner1, Andreas Egger2, Rotraut Merkle5, Werner Sitte2, Joachim Maier5, Werner Grogger1 1Institute of Electron Microscopy and Nanoanalysis (FELMI), Graz University of Technology & Graz Centre for Electron Microscopy (ZFE), Graz, Austria. 2Chair of Physical Chemistry, Montanuniversitaet Leoben, Leoben, Austria. 3University Service Centre for Transmission Electron Microscopy (USTEM), TU Wien, Vienna, Austria. 4Gatan Inc., Pleasanton, USA. 5Max Planck Institute for Solid State Research, Stuttgart, Germany Abstract Text Within this abstract we present a high-resolution chemical analysis of the second order Ruddlesden-Popper phase Ba1.1La1.9Fe2O7 using EDX and EELS in STEM, as well as simulations for quantifying the lanthanum and barium concentration on each atom column. Lanthanum barium ferrates have auspicious properties as triple conducting oxides (proton-, oxygen ion- and electron-conducting) and therefore are promising new materials for future applications in protonic ceramic fuel cells, electrolyser cells or membranes for hydrogen separation [1]. However, mass and charge transport as well as defect chemistry are only partially understood at this point. These properties are influenced by the -
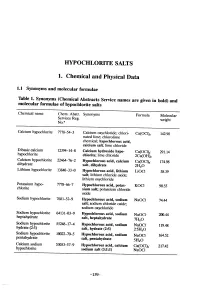
Hypochlorite Salts, As Weil As Chlorine Itself, in Aqueous Solution Produce Equilbrium Mixures of Hypochlorous Acid, Hypochlorite Ion and Chlorine
HYOCHLORITE SALTS 1. Chemical and Physical Data 1.1 Synonyms and molecular rormulae Table 1. Synonyms (Chemical Abstracts Service names are given in bold) and molecular rormulae or hyphlorite salts Chemical name Chem. Abstr. Synonyms Formula Molecular Servces Reg. weight No.u Calcium hyphlorite 7778-54-3 Calcium oxychloride; chlori- Ca(OCI)2 142.98 nated lime; chlorolime chemical; hypochlorous acid, calcium salt; lime chloride Dibasic calcium 12394-14-8 Calcium hydroxide hyp Ca(OCI)2" 291.14 hyphlorite chlonte; lime chloride 2Ca(OH)2 Calcium hyphlorite 22476-2 Hypochlorous acid, calcium Ca(OCI)2" 174.98 dihydrate salt, dihydrate 2H2O Lithium hyphlorite 1384-33-0 Hypchlorous acid, lithium LiOCI 58.39 salt; lithium chloride oxide; lithium oxychloride Potasium hyp 7778-6-7 Hypchlorous acid, potas- KOCI 90.55 chlorite sium salt; potasium chloride oxide Sodium hyphlorite 7681-52-9 Hyphlorous acid, sodium NaOCl 74.44 salt; soium chloride oxide; soium oxychloride Soium hyphlorite 64131-03-9 Hypchlorous acid, sodium heptahydrate NaOCl- 20.44 salt, heptahydrate 7H2O Sodium hyphlorite 5524-17-4 Hyphlorous acid, sodium NaOCI. 119.48 hydrate (2:5) salt, hydrate (2:5) 2-5H2O Sodium hyphlorite 10022-70-5 Hyphlorous acid, sodium NaOCI" 164.52 pentahydrate salt, pentahydrate 5H2O Calcium soium 53053-57-9 Hypchlorous acid, calcium Ca(OCI)2- 217.42 hyphlorite sodium salt (3:1:1) NaOCI -159- 160 lARe MONOGRAHS VOLUME 52 1.2 Chernical and physical properties or the pure substances From Weast (1989) unless otherwise specified Calciurn hyphlorite (a) Description: White powder or flat plates (b) Melting-point: Decomposes at 100°C (c) Density Specific gravity = 2.35 (d) Solubility: Soluble in cold water, 21.4% soluble at 25°C (Wojtowicz, 1979); insoluble in ethanol (e) Stability: Solid form decomposes exothermically when heated to 175°C, releasing oxygen (Mannsvile Chemical Products Corp., 1987). -

EPDM & FKM Chemical Resistance Guide
EPDM & FKM Chemical Resistance Guide SECOND EDITION EPDM & FKM CHEMICAL RESISTANCE GUIDE Elastomers: Ethylene Propylene (EPDM) Fluorocarbon (FKM) Chemical Resistance Guide Ethylene Propylene (EPDM) & Fluorocarbon (FKM) 2nd Edition © 2020 by IPEX. All rights reserved. No part of this book may be used or reproduced in any manner whatsoever without prior written permission. For information contact: IPEX, Marketing, 1425 North Service Road East, Oakville, Ontario, Canada, L6H 1A7 ABOUT IPEX At IPEX, we have been manufacturing non-metallic pipe and fittings since 1951. We formulate our own compounds and maintain strict quality control during production. Our products are made available for customers thanks to a network of regional stocking locations from coast-to-coast. We offer a wide variety of systems including complete lines of piping, fittings, valves and custom-fabricated items. More importantly, we are committed to meeting our customers’ needs. As a leader in the plastic piping industry, IPEX continually develops new products, modernizes manufacturing facilities and acquires innovative process technology. In addition, our staff take pride in their work, making available to customers their extensive thermoplastic knowledge and field experience. IPEX personnel are committed to improving the safety, reliability and performance of thermoplastic materials. We are involved in several standards committees and are members of and/or comply with the organizations listed on this page. For specific details about any IPEX product, contact our customer service department. INTRODUCTION Elastomers have outstanding resistance to a wide range of chemical reagents. Selecting the correct elastomer for an application will depend on the chemical resistance, temperature and mechanical properties needed. Resistance is a function both of temperatures and concentration, and there are many reagents which can be handled for limited temperature ranges and concentrations. -

Potassium Permanganate MSDS
He a lt h 2 0 Fire 0 1 0 Re a c t iv it y 0 Pe rs o n a l Pro t e c t io n J Material Safety Data Sheet Potassium permanganate MSDS Section 1: Chemical Product and Company Identification Product Name: Potassium permanganate Contact Information: Catalog Codes: SLP4912, SLP3892, SLP1075 Sciencelab.com, Inc. 14025 Smith Rd. CAS#: 7722-64-7 Houston, Texas 77396 RTECS: SD6475000 US Sales: 1-800-901-7247 International Sales: 1-281-441-4400 TSCA: TSCA 8(b) inventory: Potassium permanganate Order Online: ScienceLab.com CI#: Not available. CHEMTREC (24HR Emergency Telephone), call: Synonym: Potassium Permanganate, Biotech Grade 1-800-424-9300 Chemical Name: Potassium Permanganate International CHEMTREC, call: 1-703-527-3887 Chemical Formula: KMnO4 For non-emergency assistance, call: 1-281-441-4400 Section 2: Composition and Information on Ingredients Composition: Name CAS # % by Weight Potassium permanganate 7722-64-7 100 Toxicological Data on Ingredients: Potassium permanganate, Biotech: ORAL (LD50): Acute: 1090 mg/kg [Rat]. 2157 mg/kg [Mouse]. Section 3: Hazards Identification Potential Acute Health Effects: Hazardous in case of skin contact (irritant), of eye contact (irritant), of ingestion, of inhalation. Slightly hazardous in case of skin contact (permeator). Possibly corrosive to eyes and skin. The amount of tissue damage depends on length of contact. Eye contact can result in corneal damage or blindness. Skin contact can produce inflammation and blistering. Inhalation of dust will produce irritation to gastro-intestinal or respiratory tract, characterized by burning, sneezing and coughing. Severe over-exposure can produce lung damage, choking, unconsciousness or death.